Chemical combustion is a rapid exothermic reaction between a fuel and an oxidant, releasing heat and light energy. This process is essential in many applications, from powering engines to generating electricity, where controlled combustion ensures efficiency and safety. Explore the article to understand how chemical combustion impacts your daily life and industry.
Table of Comparison
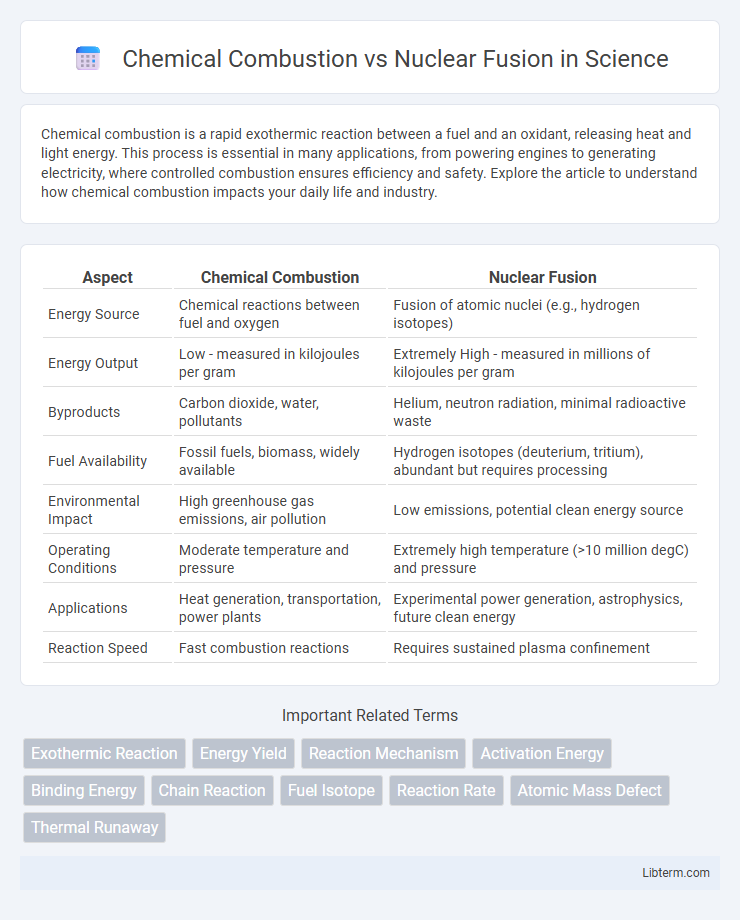
| Aspect | Chemical Combustion | Nuclear Fusion |
|---|---|---|
| Energy Source | Chemical reactions between fuel and oxygen | Fusion of atomic nuclei (e.g., hydrogen isotopes) |
| Energy Output | Low - measured in kilojoules per gram | Extremely High - measured in millions of kilojoules per gram |
| Byproducts | Carbon dioxide, water, pollutants | Helium, neutron radiation, minimal radioactive waste |
| Fuel Availability | Fossil fuels, biomass, widely available | Hydrogen isotopes (deuterium, tritium), abundant but requires processing |
| Environmental Impact | High greenhouse gas emissions, air pollution | Low emissions, potential clean energy source |
| Operating Conditions | Moderate temperature and pressure | Extremely high temperature (>10 million degC) and pressure |
| Applications | Heat generation, transportation, power plants | Experimental power generation, astrophysics, future clean energy |
| Reaction Speed | Fast combustion reactions | Requires sustained plasma confinement |
Introduction to Chemical Combustion and Nuclear Fusion
Chemical combustion involves the rapid oxidation of a fuel source, typically hydrocarbons, releasing energy through chemical bonds in the presence of oxygen. Nuclear fusion, by contrast, fuses atomic nuclei, such as hydrogen isotopes, to form heavier elements, releasing significantly higher energy per reaction. While chemical combustion powers everyday engines and heating systems, nuclear fusion promises vast energy production with minimal environmental impact.
Fundamental Principles of Combustion and Fusion
Chemical combustion involves a reaction between a fuel and an oxidant, typically oxygen, producing heat and light through the breaking and forming of chemical bonds in molecules. Nuclear fusion, on the other hand, is a process where atomic nuclei combine at extremely high temperatures and pressures, releasing energy by converting mass into energy as described by Einstein's equation E=mc2. The fundamental difference lies in combustion relying on electron interactions within molecules, whereas fusion involves the strong nuclear force and changes in atomic nuclei.
Energy Output Comparison
Nuclear fusion releases energy millions of times greater per reaction than chemical combustion, with fusion of hydrogen isotopes producing approximately 17.6 MeV (million electron volts) per event compared to the roughly 4 eV released by burning a single molecule of methane. The energy density of fusion fuels like deuterium and tritium exceeds that of fossil fuels by several orders of magnitude, enabling far more power generation in much smaller fuel quantities. This vast difference in energy output underscores fusion's potential as a revolutionary energy source compared to conventional chemical combustion processes.
Reactant Materials and Availability
Chemical combustion relies on readily available reactants such as hydrocarbons and oxygen, which are abundant and easily sourced from fossil fuels and the atmosphere. Nuclear fusion requires isotopes like deuterium and tritium, with deuterium readily extracted from seawater but tritium produced in limited quantities through neutron bombardment of lithium. The scarcity of tritium and the challenges in handling fusion fuel contrast sharply with the widespread availability and simplicity of chemical combustion materials.
Byproducts and Environmental Impact
Chemical combustion produces carbon dioxide, nitrogen oxides, and particulate matter, contributing significantly to air pollution and climate change through greenhouse gas emissions. Nuclear fusion generates helium and minimal radioactive waste, presenting a cleaner energy alternative with a minimal environmental footprint. The long-lived radioactive byproducts of fission are drastically reduced in fusion, making its waste management simpler and enhancing overall sustainability.
Safety Considerations in Each Process
Chemical combustion involves the reaction of substances with oxygen, releasing energy through rapid oxidation, but it produces harmful emissions and carries risks of fire and explosions, necessitating strict safety protocols. Nuclear fusion fuses atomic nuclei at extremely high temperatures, offering a cleaner energy source with minimal radioactive waste, though it requires robust containment systems to manage plasma stability and prevent radiation leaks. Safety in chemical combustion focuses on controlling flammable materials and emissions, while nuclear fusion demands advanced engineering to maintain controlled fusion reactions and shield radiation exposure.
Technological Applications of Combustion and Fusion
Chemical combustion powers internal combustion engines, gas turbines, and industrial furnaces, playing a crucial role in transportation, electricity generation, and manufacturing. Nuclear fusion, still in experimental stages, promises a future of cleaner energy with projects like ITER and DEMO aiming to harness fusion reactors for sustainable electricity production. While combustion remains integral to current energy infrastructure, fusion holds potential for revolutionizing power generation with minimal environmental impact.
Efficiency and Energy Conversion
Chemical combustion converts roughly 20-40% of the fuel's stored chemical energy into useful work, with significant losses as heat and emissions, making it less efficient for large-scale energy production. Nuclear fusion promises energy conversion efficiencies exceeding 70%, as it releases immense energy by merging atomic nuclei, surpassing the energy output of chemical bonds by millions of times. The high efficiency of fusion results from the conversion of mass to energy via Einstein's equation E=mc2, enabling vastly greater energy density than the electron rearrangements involved in chemical combustion.
Current and Future Developments
Chemical combustion relies on oxidation reactions involving fuel and oxygen, producing energy with limited efficiency and high carbon emissions, while nuclear fusion harnesses atomic nuclei combining under extreme temperatures for vastly greater energy output and minimal environmental impact. Recent advancements in magnetic confinement and inertial confinement methods have brought nuclear fusion closer to viable energy production, with experimental reactors like ITER and NIF progressing toward net-positive energy generation. Future developments emphasize improving plasma stability, sustained reaction duration, and cost-effective reactor materials to enable commercial fusion power plants, potentially revolutionizing clean energy worldwide.
Conclusion: The Future of Energy Production
Nuclear fusion offers a far more sustainable and powerful energy source compared to chemical combustion due to its higher energy density and minimal greenhouse gas emissions. Advances in fusion reactor technologies, such as tokamaks and inertial confinement systems, are accelerating the potential for clean, virtually limitless energy production. Chemical combustion remains dominant for now, but fusion could revolutionize the global energy landscape by providing a long-term solution to climate change and energy security.
Chemical Combustion Infographic
 libterm.com
libterm.com