Hydrophilic substances readily attract and interact with water molecules due to their polar nature, making them essential in biological processes and product formulations. These materials enhance hydration, improve solubility, and facilitate effective chemical reactions in aqueous environments. Discover how understanding hydrophilic properties can optimize your applications by reading the rest of this article.
Table of Comparison
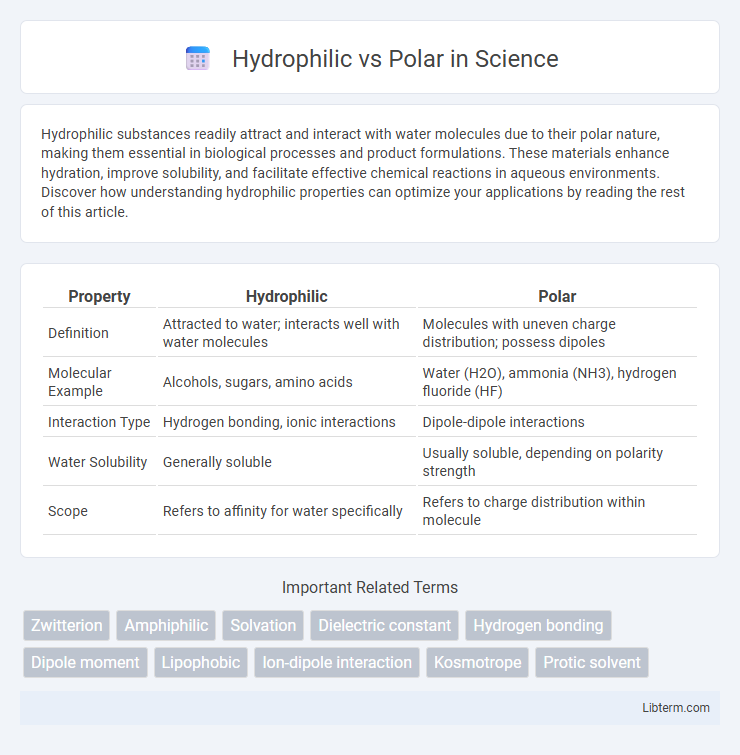
| Property | Hydrophilic | Polar |
|---|---|---|
| Definition | Attracted to water; interacts well with water molecules | Molecules with uneven charge distribution; possess dipoles |
| Molecular Example | Alcohols, sugars, amino acids | Water (H2O), ammonia (NH3), hydrogen fluoride (HF) |
| Interaction Type | Hydrogen bonding, ionic interactions | Dipole-dipole interactions |
| Water Solubility | Generally soluble | Usually soluble, depending on polarity strength |
| Scope | Refers to affinity for water specifically | Refers to charge distribution within molecule |
Understanding Hydrophilic and Polar Concepts
Hydrophilic substances possess an affinity for water due to their ability to form hydrogen bonds, typically containing polar functional groups such as hydroxyl (-OH) or amine (-NH2) groups. Polar molecules exhibit an uneven distribution of electron density, creating partial positive and negative charges that enable interactions with other polar entities and water molecules. Understanding the distinction lies in recognizing that all hydrophilic molecules are polar, but not all polar molecules are necessarily hydrophilic, as hydrophilicity specifically refers to water solubility driven by polarity and hydrogen bonding capacity.
Chemical Structure Differences
Hydrophilic molecules contain functional groups such as hydroxyl (-OH), amino (-NH2), or carboxyl (-COOH) that form hydrogen bonds with water, enhancing solubility. Polar molecules possess uneven electron distribution resulting from electronegative atoms like oxygen or nitrogen bonded to less electronegative atoms, creating dipole moments. The key chemical structure difference lies in hydrophilic groups' ability to interact directly with water molecules, while polarity refers to the overall electron density imbalance within a molecule.
Molecular Interactions Explained
Hydrophilic molecules exhibit a strong affinity for water due to their ability to form hydrogen bonds and ion-dipole interactions through polar functional groups like hydroxyl or amine groups. Polar molecules possess permanent dipole moments resulting from electronegativity differences between atoms, enabling dipole-dipole interactions that drive solubility in polar solvents such as water. The distinction lies in hydrophilicity emphasizing water compatibility via molecular interactions, while polarity describes the inherent charge distribution influencing intermolecular forces.
Hydrophilicity vs Polarity: Key Distinctions
Hydrophilicity refers to the affinity of molecules or functional groups for water, driven by their ability to form hydrogen bonds, whereas polarity measures the distribution of electrical charge within a molecule. While hydrophilic substances are often polar due to their polar functional groups, not all polar molecules exhibit hydrophilicity, especially if their polarity does not favor interactions with water. Understanding the key distinctions between hydrophilicity and polarity is crucial in fields such as chemistry and biochemistry, affecting solubility, molecular interactions, and biological function.
Examples of Hydrophilic Molecules
Hydrophilic molecules, such as glucose, amino acids, and ethanol, contain polar functional groups like hydroxyl (-OH) or amino (-NH2) groups that facilitate strong interactions with water through hydrogen bonding. These molecules differ from merely polar molecules, which may not always be hydrophilic if they cannot effectively form hydrogen bonds or dissolve in water. Understanding the specific examples of hydrophilic substances helps clarify their role in biological processes and chemical solubility.
Examples of Polar Molecules
Water (H2O), ammonia (NH3), and hydrogen chloride (HCl) are common examples of polar molecules due to their uneven distribution of electron density, resulting in partial positive and negative charges. These molecules exhibit dipole moments because of differences in electronegativity between bonded atoms, which leads to polarity. Polar molecules interact strongly with other polar or ionic substances, making them highly soluble in water and essential in various biological and chemical processes.
Role in Solubility and Solution Chemistry
Hydrophilic molecules possess polar functional groups that enable strong interactions with water molecules through hydrogen bonding, significantly enhancing their solubility in aqueous solutions. Polar compounds contain uneven charge distribution, which allows them to dissolve well in polar solvents like water by facilitating dipole-dipole interactions. The extent of hydrophilicity and polarity directly influences solvation dynamics and the stability of chemical solutions in various fields, including pharmaceuticals and biochemistry.
Biological Significance and Applications
Hydrophilic molecules, characterized by their affinity for water due to polar or charged functional groups, play critical roles in biological systems such as facilitating nutrient transport and enzyme-substrate interactions. Polar molecules, distinguished by uneven electron distribution causing dipole moments, are essential in membrane formation and cell signaling through selective permeability and receptor binding. Understanding hydrophilic and polar properties enables advancements in drug design, targeted delivery systems, and biomolecular engineering for therapeutic applications.
Common Misconceptions
Hydrophilic substances attract and interact with water due to their affinity for hydrogen bonding, whereas polar molecules have an uneven distribution of electron density but are not necessarily hydrophilic. A common misconception is that all polar molecules are hydrophilic; however, some polar molecules, like acetone, are only partially soluble in water and do not fully engage in hydrogen bonding. Understanding the distinction between polarity and hydrophilicity is essential in fields like chemistry and biology, where molecular interactions dictate solubility and function.
Summary Table: Hydrophilic vs Polar Properties
Hydrophilic substances readily interact with water due to their affinity for water molecules, often possessing polar or charged groups enabling solubility. Polar molecules contain regions of partial positive and negative charges caused by differences in electronegativity between bonded atoms, influencing their dipole moment but not necessarily ensuring hydrophilicity. The summary table highlights key distinctions: hydrophilic molecules show strong water solubility and engagement in hydrogen bonding, whereas polar molecules exhibit dipole interactions that may or may not result in water solubility depending on molecular structure and polarity strength.
Hydrophilic Infographic
 libterm.com
libterm.com