Hydrophobic materials repel water, preventing moisture absorption and maintaining dryness in various applications such as clothing, coatings, and electronics. Their unique molecular structure causes water to bead up and roll off surfaces, enhancing durability and protection. Explore the rest of the article to discover how hydrophobic technology can benefit your daily life.
Table of Comparison
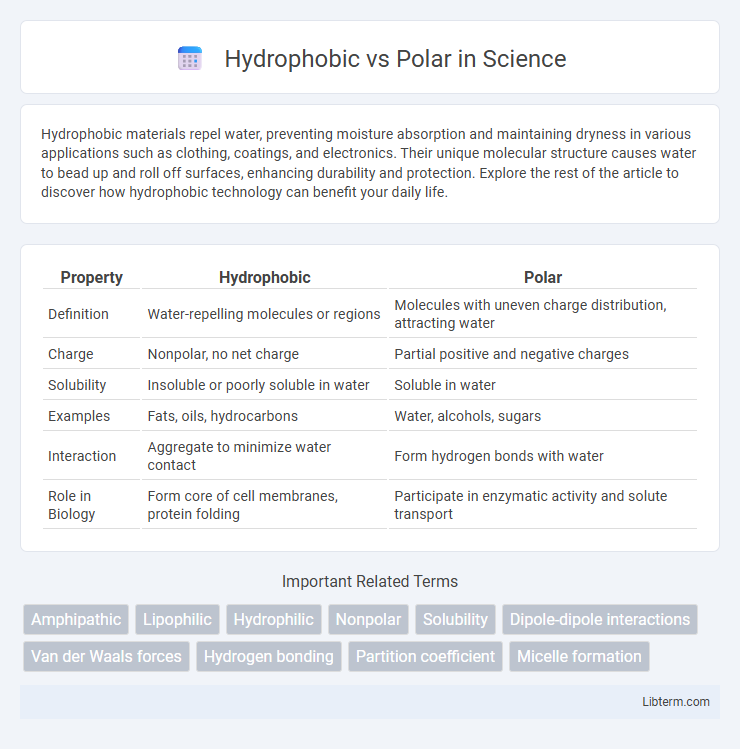
| Property | Hydrophobic | Polar |
|---|---|---|
| Definition | Water-repelling molecules or regions | Molecules with uneven charge distribution, attracting water |
| Charge | Nonpolar, no net charge | Partial positive and negative charges |
| Solubility | Insoluble or poorly soluble in water | Soluble in water |
| Examples | Fats, oils, hydrocarbons | Water, alcohols, sugars |
| Interaction | Aggregate to minimize water contact | Form hydrogen bonds with water |
| Role in Biology | Form core of cell membranes, protein folding | Participate in enzymatic activity and solute transport |
Understanding Hydrophobic and Polar Molecules
Hydrophobic molecules repel water due to nonpolar covalent bonds that lack partial charges, causing them to cluster away from aqueous environments. Polar molecules possess uneven electron distribution, resulting in partial positive and negative charges that enable interaction and solubility in water through hydrogen bonding. Understanding the differential behavior of hydrophobic and polar molecules is crucial in biochemistry for explaining membrane formation, protein folding, and molecular interactions in cellular environments.
Key Differences Between Hydrophobic and Polar Compounds
Hydrophobic compounds repel water due to their nonpolar molecular structures, resulting in low solubility in aqueous environments. In contrast, polar compounds possess unevenly distributed electric charges, enabling them to form hydrogen bonds and dissolve readily in water. These key differences influence their behavior in biological systems, chemical reactions, and material design.
Molecular Structure: Hydrophobic vs Polar
Hydrophobic molecules consist primarily of nonpolar covalent bonds, resulting in a lack of partial charges and a tendency to repel water due to their inability to form hydrogen bonds. Polar molecules contain covalent bonds with unequal electron sharing, creating partial positive and negative charges that enable strong interactions with water through hydrogen bonding. The molecular structure of hydrophobic compounds, often rich in hydrocarbons, contrasts with polar molecules that frequently include electronegative atoms such as oxygen, nitrogen, or sulfur, crucial for their solubility and reactivity in aqueous environments.
Chemical Properties and Interactions
Hydrophobic molecules are nonpolar and repel water due to their lack of charged or polar groups, causing them to cluster together in aqueous environments through van der Waals forces. Polar molecules contain partial positive and negative charges enabling hydrogen bonding and dipole-dipole interactions, which enhance their solubility in water and reactivity with other polar substances. Chemical interactions of hydrophobic substances are dominated by nonpolar covalent bonds, while polar compounds engage in ionic and hydrogen bonding, influencing their behavior in biochemical and environmental contexts.
Role in Biological Systems
Hydrophobic molecules tend to avoid water, playing a critical role in forming cell membranes by driving the self-assembly of lipid bilayers, which creates distinct intracellular environments. Polar molecules, due to their ability to form hydrogen bonds, facilitate water solubility and participate actively in biochemical reactions, such as enzyme-substrate interactions and signal transduction. The balance between hydrophobic and polar regions in proteins determines their folding, stability, and function within biological systems.
Solubility in Water: Hydrophobic vs Polar
Hydrophobic molecules exhibit low solubility in water due to their nonpolar nature, which prevents effective interactions with the polar water molecules. Polar molecules dissolve readily in water as their partial charges enable hydrogen bonding and electrostatic interactions with water. The difference in solubility is fundamentally driven by the compatibility of molecular polarity with the solvent's polarity, influencing processes like molecular transport and biochemical reactions.
Examples of Hydrophobic and Polar Molecules
Hydrophobic molecules such as oils, fats, and hydrocarbons repel water due to their nonpolar nature, lacking affinity for polar solvents. Polar molecules, including water, alcohols like ethanol, and sugars like glucose, exhibit uneven charge distribution, enabling strong interactions with other polar substances. This distinct molecular behavior influences solubility, biological membrane formation, and chemical interactions in various environments.
Impact on Membrane Formation
Hydrophobic molecules repel water and tend to aggregate, driving the self-assembly of lipid bilayers that form cellular membranes. Polar molecules interact with water through hydrogen bonds, often remaining on membrane surfaces or facilitating transport across membranes via specialized proteins. The balance between hydrophobic and polar regions in membrane lipids is crucial for membrane stability, permeability, and function in biological systems.
Applications in Industry and Science
Hydrophobic materials, repelling water, are essential in industries like textiles for water-resistant clothing and coatings that prevent corrosion and fouling in marine environments. Polar compounds, with their ability to interact with water and other polar substances, play a critical role in pharmaceuticals for drug solubility and delivery systems, as well as in chemical manufacturing for solvents and catalysts. Scientific research leverages hydrophobic and polar properties to design nanoparticles for targeted drug delivery and develop advanced materials with tailored surface interactions.
Summary: Choosing Between Hydrophobic and Polar
Choosing between hydrophobic and polar substances depends on their interaction with water and cellular environments. Hydrophobic molecules, such as lipids, repel water and are crucial for forming cell membranes and energy storage. Polar molecules, including many proteins and nucleic acids, interact readily with water, enabling critical biological functions like enzyme activity and cell signaling.
Hydrophobic Infographic
 libterm.com
libterm.com