Aldose sugars contain an aldehyde group, distinguishing them from ketose sugars that have a ketone group. They play a crucial role in energy metabolism, particularly glucose, which is an essential aldose for cellular respiration. Explore the full article to understand how aldoses impact your body's biochemical processes.
Table of Comparison
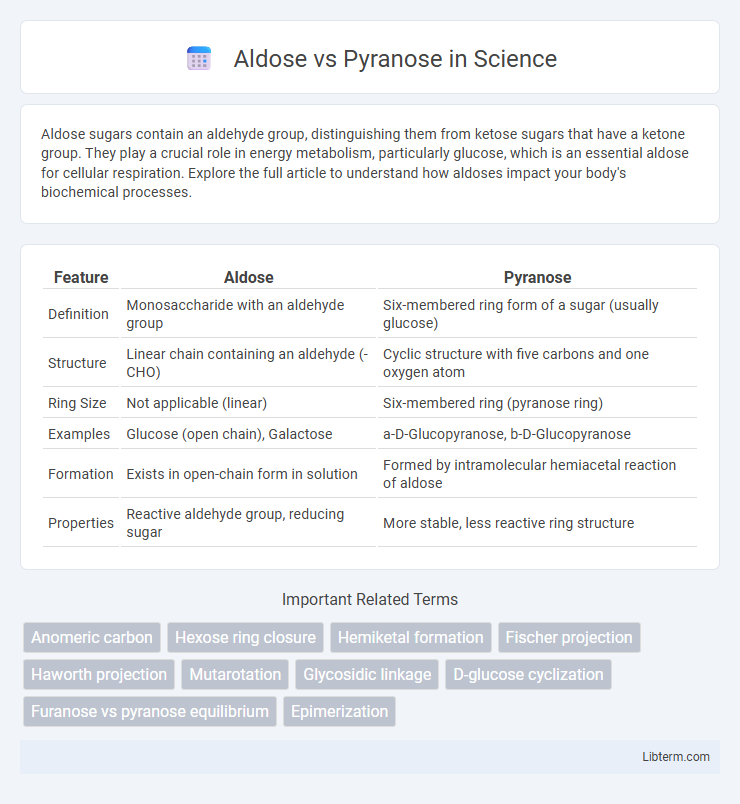
| Feature | Aldose | Pyranose |
|---|---|---|
| Definition | Monosaccharide with an aldehyde group | Six-membered ring form of a sugar (usually glucose) |
| Structure | Linear chain containing an aldehyde (-CHO) | Cyclic structure with five carbons and one oxygen atom |
| Ring Size | Not applicable (linear) | Six-membered ring (pyranose ring) |
| Examples | Glucose (open chain), Galactose | a-D-Glucopyranose, b-D-Glucopyranose |
| Formation | Exists in open-chain form in solution | Formed by intramolecular hemiacetal reaction of aldose |
| Properties | Reactive aldehyde group, reducing sugar | More stable, less reactive ring structure |
Introduction to Aldose and Pyranose
Aldoses are monosaccharides containing an aldehyde group, typically found in linear form but capable of cyclizing in aqueous solutions. Pyranoses are six-membered ring structures formed when an aldose cyclizes, creating a stable hemiacetal ring resembling pyran. This ring formation significantly impacts the sugar's chemical reactivity and physical properties, distinguishing pyranoses from their open-chain aldose counterparts.
Definition of Aldose
An aldose is a monosaccharide containing an aldehyde group (-CHO) at the first carbon atom, distinguishing it from ketoses, which have a ketone group. Aldoses commonly cyclize to form pyranose structures, six-membered rings consisting of five carbons and one oxygen atom, enhancing their stability in aqueous solutions. Glucose is a prime example of an aldose that predominantly exists in its pyranose form under physiological conditions.
Definition of Pyranose
Pyranose refers to a six-membered cyclic structure formed when an aldose sugar undergoes intramolecular hemiacetal formation, resulting in a ring consisting of five carbon atoms and one oxygen atom. This stable ring form predominates in aqueous solutions, influencing the sugar's chemical reactivity and physical properties. Understanding the pyranose configuration is crucial for studying carbohydrate behavior, enzyme interactions, and glycosidic bond formation.
Structural Differences Between Aldose and Pyranose
Aldoses are monosaccharides characterized by an aldehyde group at the carbon-1 position, while pyranoses are cyclic forms of monosaccharides containing a six-membered ring structure with five carbons and one oxygen. The structural difference lies in the open-chain form of aldoses, which contains a free aldehyde group, versus the cyclic hemiacetal form of pyranoses formed by intramolecular reaction between the aldehyde group and a hydroxyl group. This cyclization converts the linear aldehyde structure of aldoses into a stable ring, altering the chemical properties and reactivity of the sugar.
Chemical Properties Comparison
Aldoses contain an aldehyde group at the first carbon atom, which makes them more susceptible to oxidation and reduction reactions compared to pyranoses, which are cyclic hemiacetals formed from intramolecular reactions between an aldehyde or ketone and a hydroxyl group. Pyranoses exhibit enhanced stability in aqueous solutions due to their six-membered ring structure, reducing the reactivity of the aldehyde group present in their open-chain aldose form. The equilibrium between the open-chain aldose and cyclic pyranose forms significantly influences their chemical behavior, including glycosidic bond formation and mutarotation rates.
Formation Mechanisms of Aldose and Pyranose
Aldose formation involves the presence of an aldehyde group at the terminal carbon, enabling the molecule to remain in a linear form before cyclization. Pyranose forms through intramolecular hemiacetal formation when the hydroxyl group on the fifth carbon attacks the aldehyde carbon, resulting in a six-membered ring structure. This cyclization stabilizes the sugar by converting the reactive aldehyde into a cyclic hemiacetal, typical in aldose sugars like glucose.
Biological Roles and Functions
Aldoses, as simple sugars with an aldehyde group, serve as fundamental energy sources and metabolic intermediates in cellular respiration and biosynthesis, primarily in their linear form. Pyranoses, representing the cyclic six-membered ring structure formed by intramolecular hemiacetal reactions of aldoses, are crucial for structural stability and recognition in biological systems, including the formation of glycosidic bonds in polysaccharides. The prevalence of pyranose forms in carbohydrates like glucose influences enzymatic specificity, energy storage, and cell signaling pathways across diverse organisms.
Examples of Aldose and Pyranose Sugars
Glucose exemplifies an aldose sugar with an aldehyde functional group, while fructose is a key ketose sugar often involved in pyranose ring formation. The pyranose form of glucose predominates in solution, creating a six-membered ring structure that enhances its stability and biological function. Mannose and galactose also form pyranose rings, showcasing the common cyclic structures among aldose sugars in carbohydrate chemistry.
Analytical Methods for Differentiation
Analytical methods such as Nuclear Magnetic Resonance (NMR) spectroscopy effectively differentiate aldose and pyranose forms by identifying distinct chemical shifts and coupling constants specific to their ring structures. Mass spectrometry combined with chromatographic techniques like High-Performance Liquid Chromatography (HPLC) allows separation and detection of aldoses and pyranoses based on molecular weight and polarity variations. Infrared (IR) spectroscopy can also distinguish functional groups unique to aldose and pyranose configurations, aiding in their accurate identification.
Summary of Key Distinctions
Aldoses are monosaccharides containing an aldehyde group, while pyranoses are cyclic forms of sugars characterized by a six-membered ring structure. Aldoses typically exist in linear form but cyclize into pyranose rings through intramolecular hemiacetal formation involving the aldehyde carbon and a hydroxyl group. The transition from aldose to pyranose influences the sugar's chemical reactivity and stereochemistry, critical for biological functions and carbohydrate metabolism.
Aldose Infographic
 libterm.com
libterm.com