A holoenzyme is a complete, biologically active enzyme consisting of an apoenzyme and its essential cofactor or coenzyme. This combination is critical for catalyzing specific biochemical reactions efficiently within cells. Explore the rest of the article to understand how holoenzymes function and their importance in various metabolic processes.
Table of Comparison
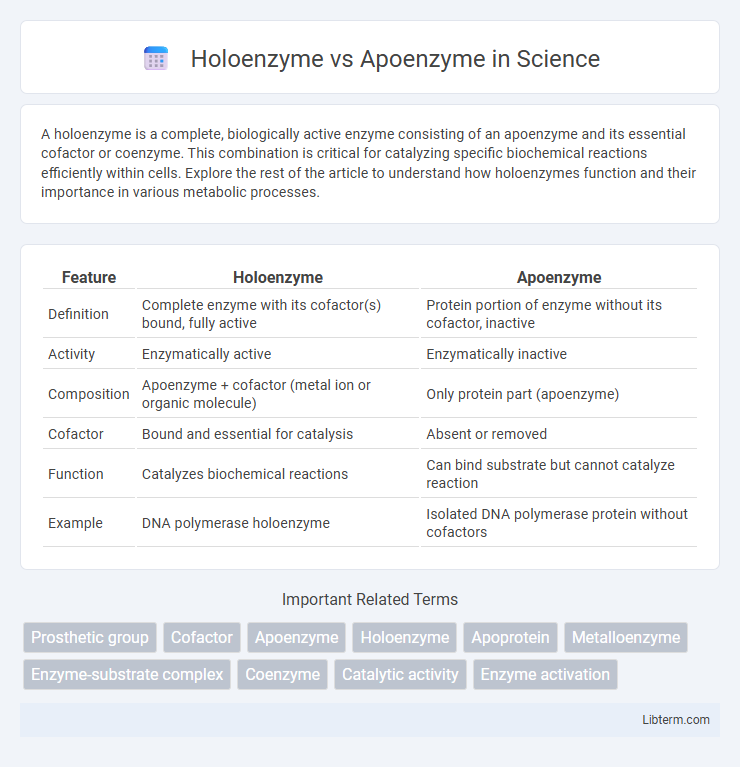
| Feature | Holoenzyme | Apoenzyme |
|---|---|---|
| Definition | Complete enzyme with its cofactor(s) bound, fully active | Protein portion of enzyme without its cofactor, inactive |
| Activity | Enzymatically active | Enzymatically inactive |
| Composition | Apoenzyme + cofactor (metal ion or organic molecule) | Only protein part (apoenzyme) |
| Cofactor | Bound and essential for catalysis | Absent or removed |
| Function | Catalyzes biochemical reactions | Can bind substrate but cannot catalyze reaction |
| Example | DNA polymerase holoenzyme | Isolated DNA polymerase protein without cofactors |
Introduction to Holoenzyme and Apoenzyme
Holoenzymes are complete, catalytically active enzyme complexes formed when an apoenzyme binds to its necessary cofactor or coenzyme, enabling it to perform its biological function. Apoenzymes represent the inactive protein portion of enzymes, lacking the essential non-protein component required for enzymatic activity. Understanding the distinction between holoenzymes and apoenzymes is crucial for exploring enzyme mechanisms and regulatory processes in biochemical reactions.
Definition of Holoenzyme
A holoenzyme is a complete, catalytically active enzyme consisting of an apoenzyme bound to its required cofactor or coenzyme, essential for its biological function. In contrast, an apoenzyme is the protein component alone, inactive without the necessary non-protein molecule. The holoenzyme's structure enables substrate binding and catalysis, playing a crucial role in metabolic reactions and enzymatic specificity.
Definition of Apoenzyme
Apoenzyme refers to the protein portion of an enzyme, excluding its necessary cofactor or coenzyme, making it inactive on its own. In contrast, the holoenzyme is the complete, catalytically active enzyme consisting of the apoenzyme combined with its specific cofactor or coenzyme. The interaction between apoenzyme and cofactor is essential for the enzyme's biological activity and substrate specificity.
Structural Differences
Holoenzymes consist of both the apoenzyme (protein portion) and its necessary cofactor or coenzyme, enabling enzymatic activity, whereas apoenzymes lack these non-protein components and are structurally incomplete for catalysis. The structural difference lies primarily in the presence of the cofactor binding sites occupied in holoenzymes, altering the conformation of the active site for substrate interaction. This structural integration of cofactors in holoenzymes is crucial for stabilizing enzyme configuration and facilitating biochemical reactions.
Functional Distinctions
Holoenzymes are complete, biologically active enzymes consisting of an apoenzyme (protein component) and its essential cofactor, enabling catalytic activity. Apoenzymes lack their cofactors and remain inactive, requiring cofactor binding to form functional holoenzymes. The presence or absence of the cofactor distinguishes the enzymatic function, with holoenzymes catalyzing biochemical reactions and apoenzymes serving as inactive precursors.
Role of Cofactors in Enzyme Activity
Holoenzymes consist of an apoenzyme combined with essential cofactors, which are non-protein molecules or ions crucial for enzymatic activity and proper catalytic function. Apoenzymes alone are inactive, as they lack these cofactors such as metal ions or organic molecules (coenzymes) necessary for substrate binding and conversion. The presence of cofactors induces conformational changes in the apoenzyme, facilitating the formation of the active site and enabling efficient substrate processing during metabolic reactions.
Examples of Holoenzymes and Apoenzymes
Holoenzymes are active enzyme complexes consisting of a protein component (apoenzyme) and a non-protein cofactor or coenzyme, such as DNA polymerase which requires divalent metal ions like Mg2+ for activity. Examples of holoenzymes include RNA polymerase in bacteria, which incorporates multiple subunits and cofactors, and pyruvate dehydrogenase complex containing TPP and lipoamide as coenzymes. Apoenzymes, on the other hand, lack their necessary cofactors and are inactive; for instance, inactive glutamate dehydrogenase without its NAD+ coenzyme or inactive carbonic anhydrase missing its zinc ion are classified as apoenzymes.
Biological Significance
Holoenzymes represent the biologically active form of enzymes, consisting of an apoenzyme combined with its necessary cofactor, allowing precise catalysis of biochemical reactions essential for metabolism. Apoenzymes alone are inactive protein moieties requiring specific cofactors such as metal ions or organic molecules to restore enzymatic function and regulate metabolic pathways. The interplay between holoenzyme and apoenzyme forms is critical for controlling enzyme activity, substrate specificity, and cellular response to environmental changes.
Clinical and Industrial Relevance
Holoenzymes, as fully active enzyme complexes with their necessary cofactors, are critical in clinical diagnostics and industrial biocatalysis for precise biochemical reactions and efficient substrate conversion. Apoenzymes, lacking cofactors and inactive alone, are significant in drug development for targeting enzyme activation pathways and in industrial processes requiring controlled enzyme activation. Understanding the structural differences between holoenzymes and apoenzymes enables improved enzyme engineering for therapeutic enzyme replacement therapies and optimized industrial enzyme formulations.
Summary and Key Takeaways
Holoenzyme is the complete, active enzyme consisting of the apoenzyme and its necessary cofactor or coenzyme, essential for catalytic activity, whereas the apoenzyme is the inactive protein portion lacking its cofactor. The apoenzyme requires binding with a specific cofactor (metal ion) or coenzyme (organic molecule) to form the holoenzyme and exhibit enzymatic function. Understanding the distinction between holoenzyme and apoenzyme is crucial for enzyme kinetics, drug targeting, and biochemical regulation.
Holoenzyme Infographic
 libterm.com
libterm.com