Ketose sugars, such as fructose, contain a ketone group, distinguishing them from aldose sugars that have an aldehyde group. These carbohydrates play crucial roles in metabolism and energy production within your body. Explore the article to understand the importance and functions of ketoses in biological systems.
Table of Comparison
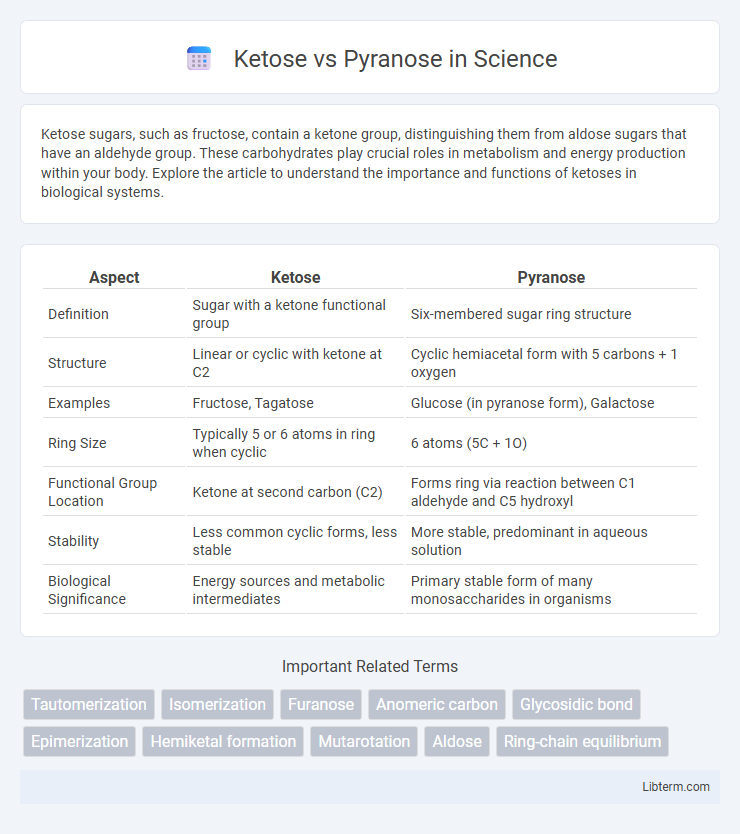
| Aspect | Ketose | Pyranose |
|---|---|---|
| Definition | Sugar with a ketone functional group | Six-membered sugar ring structure |
| Structure | Linear or cyclic with ketone at C2 | Cyclic hemiacetal form with 5 carbons + 1 oxygen |
| Examples | Fructose, Tagatose | Glucose (in pyranose form), Galactose |
| Ring Size | Typically 5 or 6 atoms in ring when cyclic | 6 atoms (5C + 1O) |
| Functional Group Location | Ketone at second carbon (C2) | Forms ring via reaction between C1 aldehyde and C5 hydroxyl |
| Stability | Less common cyclic forms, less stable | More stable, predominant in aqueous solution |
| Biological Significance | Energy sources and metabolic intermediates | Primary stable form of many monosaccharides in organisms |
Introduction to Ketose and Pyranose
Ketose are monosaccharides characterized by a ketone group, differentiating them from aldose sugars. Pyranose refer to six-membered ring structures formed when monosaccharides cyclize, typically resembling a pyran ring. Understanding the structural difference between linear ketose forms and cyclic pyranose forms is essential in carbohydrate chemistry and metabolism.
Structural Overview of Ketoses
Ketoses are monosaccharides characterized by the presence of a ketone functional group, typically located at the second carbon atom, unlike aldoses which contain an aldehyde group. Structurally, ketoses often form five-membered ring structures known as furanoses but can also cyclize into six-membered pyranose rings depending on the intramolecular reaction between the ketone group and a hydroxyl group. This cyclization alters their stereochemistry, influencing their biochemical properties and roles in metabolic pathways such as glycolysis and the pentose phosphate pathway.
Structural Overview of Pyranoses
Pyranoses are six-membered ring structures formed when a sugar's carbonyl group reacts intramolecularly with a hydroxyl group, creating a stable hemiacetal. This ring includes five carbon atoms and one oxygen atom, resembling the structure of pyran, and commonly occurs in aldoses like glucose. The chair and boat conformations of pyranoses influence their stereochemistry and reactivity, critical in biological processes such as glycosidic bond formation.
Chemical Differences: Ketose vs Pyranose
Ketose and pyranose differ fundamentally in their chemical structures; ketoses contain a ketone functional group typically at the second carbon atom, whereas pyranoses are cyclic forms of sugars characterized by a six-membered ring that includes five carbon atoms and one oxygen atom. Ketoses exist predominantly in an open-chain form with a carbonyl group, while pyranoses result from intramolecular cyclization forming a hemiacetal ring structure. The ring closure in pyranoses alters the chemical reactivity and stereochemistry compared to the linear ketose form, influencing their biological roles and enzymatic interactions.
Biological Roles of Ketose Sugars
Ketose sugars, characterized by a ketone functional group, play essential biological roles in energy metabolism, notably in the glycolytic pathway where fructose-6-phosphate acts as a key intermediate. Unlike pyranose forms, ketoses such as fructose exist predominantly in furanose ring structures that facilitate specific enzyme recognition and catalysis. Ketose sugars also contribute to nucleotide sugar biosynthesis and cellular signaling processes, underscoring their critical function in metabolic regulation and biochemical transformations.
Significance of Pyranose Forms in Nature
Pyranose forms are predominant in nature due to their enhanced stability and structural versatility, providing the basis for many essential biological molecules like glucose and galactose. Unlike ketose forms, pyranoses adopt a six-membered ring structure that facilitates more stable glycosidic linkages critical for energy storage and cell wall construction. This configuration also supports enzymatic recognition and interaction, making pyranose rings fundamental in metabolic pathways and polysaccharide assembly.
Common Examples of Ketoses and Pyranoses
Ketoses commonly include fructose, sorbose, and tagatose, characterized by a ketone group typically at the second carbon, while pyranoses refer to six-membered ring forms of sugars such as glucose, galactose, and mannose. Fructose primarily exists in a furanose form but can cyclize into a pyranose ring under certain conditions, whereas glucose predominantly adopts the pyranose ring structure due to its stable six-membered ring formation. Understanding these structural distinctions is crucial for studying carbohydrate chemistry, metabolism, and the functional roles of these sugars in biological systems.
Functional Implications in Biochemistry
Ketose sugars, characterized by a ketone group typically at the second carbon, exhibit distinct reactivity compared to pyranose forms, which are six-membered cyclic hemiacetals commonly derived from aldoses. The functional implications in biochemistry include ketoses' enhanced ability to participate in isomerization and phosphorylation reactions critical for metabolic pathways like glycolysis. Pyranose forms provide structural stability important in cell wall polysaccharides and glycoproteins, influencing molecular recognition and enzymatic interactions within biological systems.
Analytical Methods for Identifying Ketose and Pyranose
Analytical methods for identifying ketose and pyranose primarily rely on spectroscopic and chromatographic techniques such as NMR spectroscopy, which differentiates these isomers based on distinct chemical shifts and coupling patterns of anomeric protons. Mass spectrometry coupled with chromatography enables precise molecular weight determination and separation of ketose sugars from pyranose ring forms, discerned by differences in retention times and fragmentation patterns. Infrared spectroscopy can detect characteristic functional groups, while optical rotation measurements provide insights into stereochemistry, aiding the differentiation of ketose from pyranose structures in complex mixtures.
Key Takeaways: Ketose vs Pyranose
Ketose sugars contain a ketone functional group, typically at the second carbon atom, distinguishing them structurally from aldoses. Pyranose refers to the six-membered ring form of monosaccharides, which can include both ketoses and aldoses cyclized through intramolecular hemiacetal or hemiketal formation. Understanding ketose versus pyranose highlights the difference between linear functional group placement and cyclic ring structure, crucial for biochemical recognition and reactivity.
Ketose Infographic
 libterm.com
libterm.com