Polymerization is the chemical process that combines small molecules called monomers into large, complex molecules known as polymers, which form the basis of many materials including plastics and resins. This reaction can proceed through different mechanisms such as addition or condensation polymerization, each creating polymers with unique properties suited for various industrial applications. Discover how understanding polymerization can enhance your knowledge of material science by exploring the detailed processes and uses in the rest of this article.
Table of Comparison
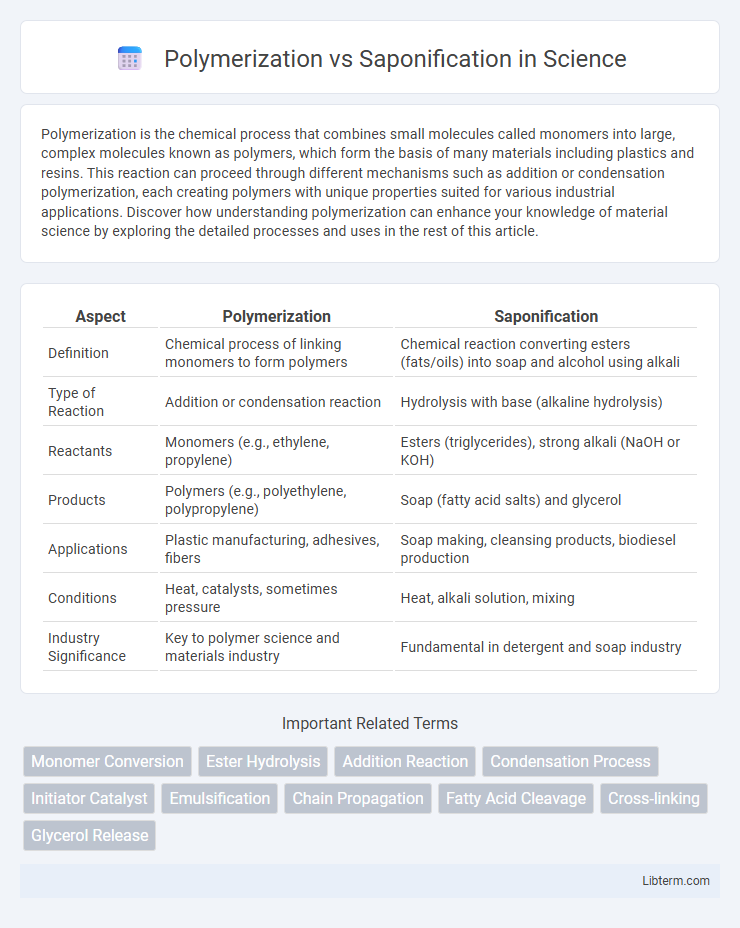
| Aspect | Polymerization | Saponification |
|---|---|---|
| Definition | Chemical process of linking monomers to form polymers | Chemical reaction converting esters (fats/oils) into soap and alcohol using alkali |
| Type of Reaction | Addition or condensation reaction | Hydrolysis with base (alkaline hydrolysis) |
| Reactants | Monomers (e.g., ethylene, propylene) | Esters (triglycerides), strong alkali (NaOH or KOH) |
| Products | Polymers (e.g., polyethylene, polypropylene) | Soap (fatty acid salts) and glycerol |
| Applications | Plastic manufacturing, adhesives, fibers | Soap making, cleansing products, biodiesel production |
| Conditions | Heat, catalysts, sometimes pressure | Heat, alkali solution, mixing |
| Industry Significance | Key to polymer science and materials industry | Fundamental in detergent and soap industry |
Introduction to Polymerization and Saponification
Polymerization is a chemical process where small molecules called monomers chemically bond to form long-chain polymers, essential in producing plastics and resins. Saponification is a specific chemical reaction where triglycerides react with a strong base, typically sodium hydroxide, to yield glycerol and soap, crucial in soap manufacturing. Understanding these fundamental reactions provides insight into their distinct industrial applications in materials science and cosmetics.
Defining Polymerization: Basics and Mechanisms
Polymerization is a chemical process where small molecules called monomers chemically bond to form long chains or networks, creating polymers with distinct physical properties. This process occurs through mechanisms such as addition polymerization, where monomers add sequentially without byproducts, or condensation polymerization, which involves the elimination of small molecules like water. Understanding the types of polymerization and their mechanisms is essential for developing materials ranging from plastics to resins.
Saponification Explained: An Overview
Saponification is a chemical reaction that involves the hydrolysis of esters, typically triglycerides found in fats and oils, by a strong base such as sodium hydroxide, resulting in the formation of glycerol and soap molecules. This process is fundamental in soap making, where the fatty acid salts produced act as surfactants to emulsify and remove oils and dirt. Unlike polymerization, which links monomers into large macromolecules, saponification breaks down complex fats into simpler, functional compounds essential for cleansing and industrial applications.
Key Chemical Reactions: Polymerization vs Saponification
Polymerization involves the chemical process where monomers link to form long polymer chains through reactions such as addition or condensation, crucial in producing plastics and resins. Saponification is a hydrolysis reaction where triglycerides react with a strong base, typically sodium hydroxide, to produce glycerol and soap molecules. The key difference lies in polymerization forming macromolecules via bonding monomers, while saponification breaks ester bonds in fats to create soap and alcohol.
Types of Polymerization Processes
Polymerization encompasses addition, condensation, and copolymerization processes, each producing different polymer structures through distinct mechanisms; addition polymerization involves the free radical or ionic polymerization of monomers like ethylene, while condensation polymerization combines monomers with the elimination of small molecules such as water. In contrast, saponification is a specific hydrolysis reaction primarily used to produce soap by breaking down esters in fats or oils with a strong base like sodium hydroxide. Understanding the types of polymerization highlights the versatile synthetic routes for creating diverse polymers, unlike saponification which is tailored for triglyceride decomposition.
Saponification Types and Conditions
Saponification encompasses different types primarily including cold process, hot process, and rebatch methods, each varying in reaction time and temperature conditions. Cold process saponification occurs at room temperature, preserving delicate ingredients, whereas hot process applies external heat to speed up the reaction, often finishing the soap faster. Rebatching involves melting pre-made soap and adding fresh ingredients without altering saponification chemistry, allowing customization under controlled mild heating conditions.
Industrial Applications: Comparing Uses
Polymerization is extensively applied in the production of plastics, resins, and synthetic fibers, enabling the manufacturing of durable materials used in automotive, aerospace, and packaging industries. Saponification, on the other hand, is vital in the production of soaps and detergents, where the hydrolysis of fats and oils generates surfactants for cleaning and cosmetic products. While polymerization creates large macromolecules for structural and functional applications, saponification focuses on chemical conversion for consumer goods emphasizing emulsification and cleansing properties.
Environmental Impact and Sustainability
Polymerization and saponification differ significantly in environmental impact and sustainability. Polymerization, especially involving synthetic polymers like plastics, often results in non-biodegradable waste contributing to pollution and microplastic accumulation. Saponification, a chemical process that produces biodegradable soaps from natural oils and alkalis, offers a more eco-friendly alternative with minimal environmental footprint and enhanced sustainability.
Polymerization and Saponification in Everyday Life
Polymerization plays a crucial role in everyday life by forming plastics and synthetic materials used in packaging, clothing, and household items. Saponification is essential in soap making, where fats or oils react with a strong base to produce soap molecules that clean and emulsify oils and dirt. Both chemical processes significantly impact daily routines by providing durable materials and effective cleaning solutions.
Conclusion: Distinguishing Features and Practical Implications
Polymerization involves the chemical bonding of monomers to form complex, high-molecular-weight polymers, essential for producing plastics, resins, and synthetic fibers. Saponification is a base-catalyzed hydrolysis reaction that converts fats or oils into soap and glycerol, a key process in soap manufacturing and biodiesel production. Understanding these distinct mechanisms underscores their practical implications, where polymerization drives material innovation and saponification enables soap formation and waste valorization.
Polymerization Infographic
 libterm.com
libterm.com