Cyanotype is a photographic printing process that produces striking blue images using a mixture of iron compounds. This method is valued for its simplicity and the unique, vibrant blue tones it creates, making it popular among artists and photographers seeking an alternative to traditional black-and-white prints. Explore the rest of the article to discover how you can create your own cyanotype prints and master this timeless technique.
Table of Comparison
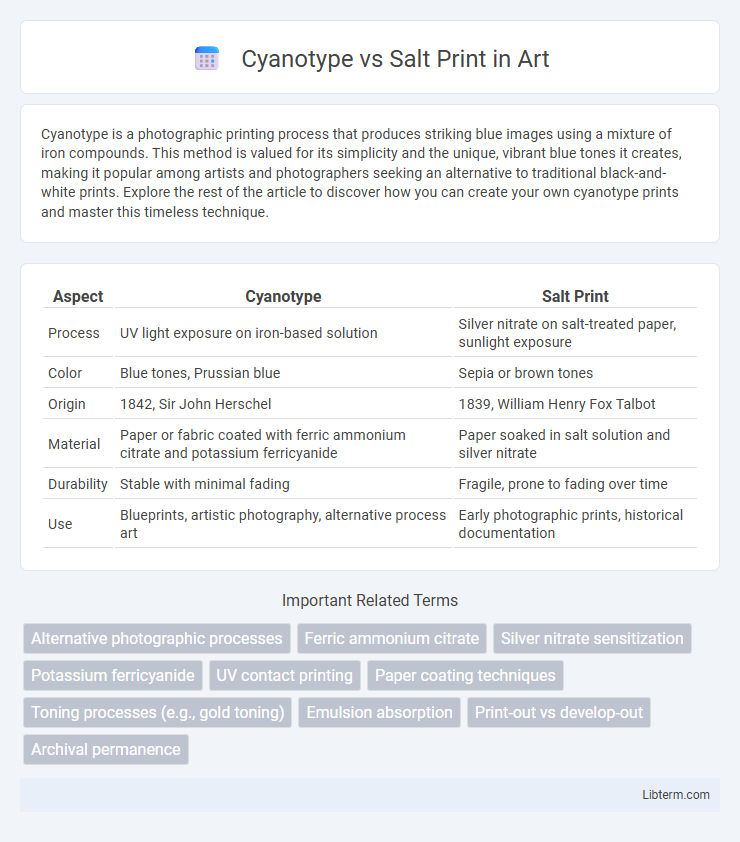
| Aspect | Cyanotype | Salt Print |
|---|---|---|
| Process | UV light exposure on iron-based solution | Silver nitrate on salt-treated paper, sunlight exposure |
| Color | Blue tones, Prussian blue | Sepia or brown tones |
| Origin | 1842, Sir John Herschel | 1839, William Henry Fox Talbot |
| Material | Paper or fabric coated with ferric ammonium citrate and potassium ferricyanide | Paper soaked in salt solution and silver nitrate |
| Durability | Stable with minimal fading | Fragile, prone to fading over time |
| Use | Blueprints, artistic photography, alternative process art | Early photographic prints, historical documentation |
Introduction to Cyanotype and Salt Print
Cyanotype and Salt Print are early photographic printing processes characterized by their unique chemical compositions and visual aesthetics. Cyanotype relies on iron salts to produce a distinctive Prussian blue image, whereas Salt Print utilizes silver salts on paper, resulting in warm, sepia-toned photographs. Both techniques played pivotal roles in the 19th-century development of photographic art and remain valued for their historical significance and artistic qualities.
Historical Background of Cyanotype
Cyanotype, developed by Sir John Herschel in 1842, is one of the earliest photographic printing processes, known for its distinctive Prussian blue color achieved through iron-based chemicals. Unlike the salt print, introduced by Henry Fox Talbot in the 1830s and relying on silver salts, cyanotype uses ferric ammonium citrate and potassium ferricyanide to create images through UV light exposure. This process gained popularity in the 19th century for scientific and botanical illustrations due to its simplicity, permanence, and cost-effectiveness compared to silver-based prints.
Historical Background of Salt Print
Salt prints, developed by William Henry Fox Talbot in the 1830s, represent one of the earliest photographic processes, using paper coated with salt and silver nitrate to create detailed monochrome images. This technique laid the groundwork for modern photography by enabling the first permanent photographic prints, preceding the widespread use of cyanotypes introduced by Sir John Herschel in 1842. Salt prints were widely popular in the mid-19th century due to their relatively straightforward process and ability to produce detailed tonal variations on paper.
Materials and Chemicals Required
Cyanotype requires ferric ammonium citrate and potassium ferricyanide mixed with water to create a photosensitive solution applied on paper or fabric, while salt printing involves soaking paper in a salt solution and then coating it with silver nitrate to develop photosensitive silver chloride. Both processes demand specific materials: Cyanotype uses UV light for exposure and washing in water, whereas salt prints require sunlight exposure, thorough washing, and often toning baths to enhance image stability. Understanding these chemical differences is crucial for selecting the appropriate method based on desired texture, tone, and archival qualities.
Step-by-Step Process: Cyanotype
The cyanotype process begins by coating paper or fabric with a light-sensitive solution of ferric ammonium citrate and potassium ferricyanide, then allowing it to dry in a dark environment. Next, the coated material is exposed to ultraviolet light with objects or negatives placed on top to create the image through light exposure. After exposure, the print is rinsed thoroughly in water to wash away unreacted chemicals, revealing a vibrant blue image characteristic of cyanotypes.
Step-by-Step Process: Salt Print
Salt print involves coating high-quality watercolor paper with a salt solution, then drying it thoroughly before applying a light-sensitive silver nitrate solution. Once dried in the dark, the paper is exposed under a negative using ultraviolet light, causing a chemical reaction that forms the image. The final print is rinsed in water to remove unexposed silver salts, fixed, and dried, resulting in a warm-toned, early photographic print with unique textural qualities.
Visual Differences and Aesthetics
Cyanotype produces vibrant blue and white images characterized by high contrast and a cool tone, while Salt Prints offer a warm sepia or brownish hue with soft gradations and nostalgic textures. The crisp, almost graphic quality of cyanotypes contrasts with the subtle, painterly appearance of salt prints, making each technique distinct in mood and visual impact. Salt Prints often reveal delicate highlights and organic imperfections, whereas cyanotypes emphasize bold outlines and structured compositions.
Longevity and Preservation
Cyanotype prints, known for their rich Prussian blue hues, exhibit exceptional stability under proper archival conditions, with longevity often surpassing 100 years when stored away from direct sunlight and humidity. Salt prints, one of the earliest photographic processes, are more susceptible to fading and deterioration due to their reliance on silver salts, requiring careful handling and storage in dark, dry environments to preserve image integrity. Preservation efforts for salt prints emphasize controlled temperature and humidity, whereas cyanotypes benefit from UV protection and minimal exposure to pollutants to maintain their vibrant tones over time.
Artistic and Modern Applications
Cyanotype offers vibrant blue hues ideal for contemporary art installations and fashion design, leveraging its waterproof qualities for durable prints on textiles and mixed media. Salt print delivers warm sepia tones favored in fine art photography and historical recreations, with its tactile surface enhancing vintage aesthetics in modern prints. Both processes enable artists to experiment with texture and tone, blending traditional techniques with innovative visual storytelling.
Choosing Between Cyanotype and Salt Print
Choosing between cyanotype and salt print depends on the desired aesthetic and technical process preferences. Cyanotype produces rich blue tones using ferric ammonium citrate and potassium ferricyanide, known for its simplicity and quick processing. Salt print offers warm sepia hues derived from silver nitrate and sodium chloride, favored for its archival quality and historical authenticity in photographic prints.
Cyanotype Infographic
 libterm.com
libterm.com