Stable isotope ratio analysis reveals subtle variations in the abundance of isotopes within a sample, providing critical insights into environmental processes, geological history, and biological systems. This technique helps trace nutrient cycles, authenticate food origins, and study climate change impacts with precision. Explore the rest of the article to understand how stable isotope ratios can uniquely inform your research or applications.
Table of Comparison
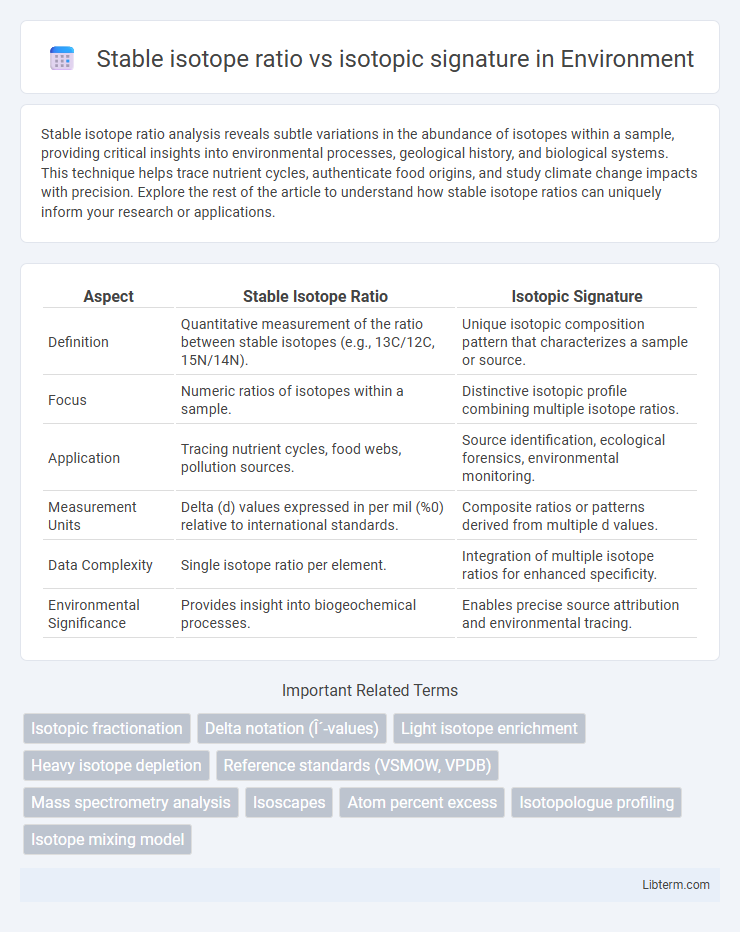
| Aspect | Stable Isotope Ratio | Isotopic Signature |
|---|---|---|
| Definition | Quantitative measurement of the ratio between stable isotopes (e.g., 13C/12C, 15N/14N). | Unique isotopic composition pattern that characterizes a sample or source. |
| Focus | Numeric ratios of isotopes within a sample. | Distinctive isotopic profile combining multiple isotope ratios. |
| Application | Tracing nutrient cycles, food webs, pollution sources. | Source identification, ecological forensics, environmental monitoring. |
| Measurement Units | Delta (d) values expressed in per mil (%0) relative to international standards. | Composite ratios or patterns derived from multiple d values. |
| Data Complexity | Single isotope ratio per element. | Integration of multiple isotope ratios for enhanced specificity. |
| Environmental Significance | Provides insight into biogeochemical processes. | Enables precise source attribution and environmental tracing. |
Introduction to Stable Isotope Ratio and Isotopic Signature
Stable isotope ratio refers to the relative abundance of isotopes of a specific element, typically expressed as a ratio of heavier to lighter isotopes, such as ^13C/^12C or ^18O/^16O. Isotopic signature represents the unique pattern of stable isotope ratios within a sample, reflecting distinct environmental, biological, or geochemical processes. Analysis of stable isotope ratios and isotopic signatures enables tracing sources, pathways, and transformations in ecological, geological, and forensic studies.
Defining Stable Isotope Ratios
Stable isotope ratios quantify the relative abundance of isotopes of a particular element, such as ^13C/^12C or ^15N/^14N, providing precise measurements for geochemical and environmental analyses. These ratios serve as key indicators in studying processes like climate change, food web dynamics, and biogeochemical cycles through their unique isotopic compositions. Unlike isotopic signatures, which encompass broader patterns or profiles of multiple isotopes within a sample, stable isotope ratios specifically refer to the numerical proportion between isotopes expressed typically in delta (d) notation.
Understanding Isotopic Signatures
Isotopic signatures represent the unique ratios of stable isotopes within a sample, reflecting specific environmental or biological processes. Stable isotope ratios, such as ^13C/^12C or ^15N/^14N, are quantitatively measured to reveal variations in isotopic signatures that serve as natural tracers in fields like ecology, geology, and forensic science. Understanding isotopic signatures enables precise interpretation of origin, diet, climate conditions, and metabolic pathways by linking stable isotope ratio patterns to specific sources and transformations.
Analytical Techniques for Isotope Ratio Measurement
Stable isotope ratio analysis utilizes mass spectrometry techniques such as isotope-ratio mass spectrometry (IRMS) to precisely measure the relative abundance of isotopes within a sample. Isotopic signature refers to the unique ratio of isotopes that characterizes a specific source or process, providing critical information in fields like geochemistry and environmental science. Advanced analytical methods, including laser-based spectroscopy and multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS), enhance accuracy and resolution in isotope ratio determination.
Applications of Stable Isotope Ratios in Science
Stable isotope ratios provide quantitative measurements of the relative abundance of isotopes in a sample, enabling precise tracing of chemical, biological, and geological processes. Isotopic signatures serve as distinct markers reflecting specific environmental, metabolic, or geochemical conditions, crucial for applications such as climate change studies, forensic analysis, and food authentication. These ratios enhance understanding of nutrient cycling, ecosystem dynamics, and paleoclimatology by revealing origin, age, and transformation pathways in diverse scientific fields.
Factors Influencing Isotopic Signatures
Stable isotope ratios reflect the relative abundance of isotopes in a sample, serving as fundamental quantitative data, while isotopic signatures represent distinctive patterns or fingerprints derived from these ratios that provide insights into environmental and biological processes. Factors influencing isotopic signatures include temperature, biological activity, source material, and kinetic or equilibrium fractionation effects during chemical reactions. Variations in isotopic signatures are driven by processes such as metabolic pathways, geographical location, and seasonal changes, which alter the stable isotope ratios in complex but predictable ways.
Case Studies: Interpreting Isotope Ratios vs. Isotopic Signatures
Case studies analyzing stable isotope ratios provide precise quantitative data on element-specific isotopic abundances, essential for tracing biogeochemical cycles and environmental changes. Interpreting isotopic signatures involves integrating these ratios with contextual information, such as source variation and fractionation processes, to accurately identify origins and mechanisms in ecological and geological systems. Distinguishing between pure isotope ratio measurements and comprehensive isotopic signatures enhances the resolution of studies in fields like hydrology, food authenticity, and paleoclimatology.
Challenges in Isotope Ratio Analysis and Signature Interpretation
Stable isotope ratio analysis faces challenges such as sample contamination, matrix effects, and instrument precision that can skew isotope measurements. Isotopic signature interpretation is complicated by overlapping source signals, diagenetic alteration, and environmental variability, which obscure the original isotopic information. Accurate integration of multi-isotope data and advanced statistical models is essential to resolve these complexities in isotope ratio and signature studies.
Advances in Stable Isotope Ratio and Signature Detection
Advances in stable isotope ratio and isotopic signature detection have significantly improved the precision and accuracy of analyzing elemental compositions in various materials. Techniques such as isotope ratio mass spectrometry (IRMS) and laser-based spectroscopy enable the differentiation of isotopic signatures at micro and nanoscale levels, facilitating applications in environmental monitoring, forensic science, and food authenticity. Enhanced sensitivity in detecting subtle variations in stable isotope ratios supports more reliable tracing of biogeochemical processes and provenance studies.
Conclusion: Choosing Between Isotope Ratios and Isotopic Signatures
Stable isotope ratios provide precise numerical values that quantify the relative abundance of isotopes, making them ideal for comparative and quantitative analyses in geochemistry and biology. Isotopic signatures integrate multiple isotope ratios to create a comprehensive profile used for source identification and process tracing in environmental and forensic studies. Selecting between isotope ratios and isotopic signatures depends on whether the study requires specific isotope quantification or a holistic isotopic pattern for complex source attribution.
Stable isotope ratio Infographic
 libterm.com
libterm.com