Trace element analysis identifies and quantifies minute concentrations of elements in materials, crucial for fields like geology, environmental science, and forensics. Techniques such as ICP-MS, XRF, and neutron activation analysis allow precise detection of these elements to uncover origins, detect contamination, or support quality control. Explore the rest of the article to understand how trace element analysis can enhance your scientific or industrial applications.
Table of Comparison
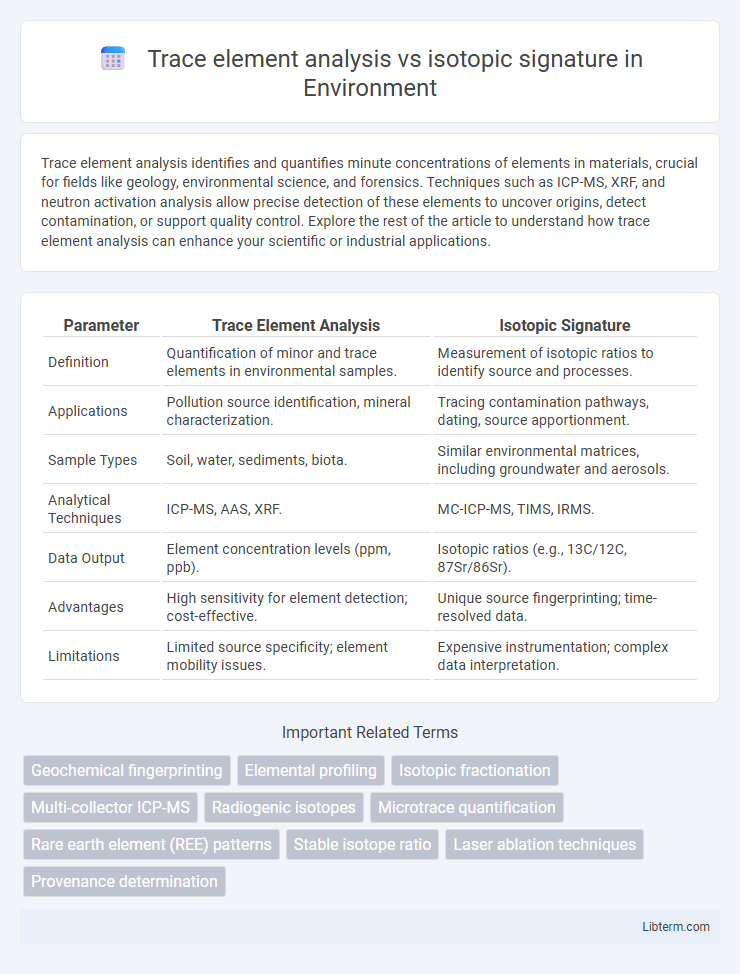
| Parameter | Trace Element Analysis | Isotopic Signature |
|---|---|---|
| Definition | Quantification of minor and trace elements in environmental samples. | Measurement of isotopic ratios to identify source and processes. |
| Applications | Pollution source identification, mineral characterization. | Tracing contamination pathways, dating, source apportionment. |
| Sample Types | Soil, water, sediments, biota. | Similar environmental matrices, including groundwater and aerosols. |
| Analytical Techniques | ICP-MS, AAS, XRF. | MC-ICP-MS, TIMS, IRMS. |
| Data Output | Element concentration levels (ppm, ppb). | Isotopic ratios (e.g., 13C/12C, 87Sr/86Sr). |
| Advantages | High sensitivity for element detection; cost-effective. | Unique source fingerprinting; time-resolved data. |
| Limitations | Limited source specificity; element mobility issues. | Expensive instrumentation; complex data interpretation. |
Introduction to Trace Element Analysis and Isotopic Signature
Trace element analysis involves measuring the concentration of minor and trace metals within a sample to reveal its geochemical composition and environmental history. Isotopic signature examines the relative abundance of isotopes present in an element, providing insights into processes such as origin, age, and alteration of materials. Both methods are essential in fields like geology, archaeology, and environmental science for distinguishing sources and understanding complex chemical interactions.
Fundamental Concepts: Trace Elements vs Isotopic Signatures
Trace element analysis involves measuring the concentration of minor elements within a sample, providing insights into its chemical composition, alteration processes, and source characteristics. Isotopic signatures focus on the ratios of stable or radioactive isotopes, revealing information about age, origin, and geochemical pathways. Both methods complement each other by combining elemental abundance with isotopic ratios to enhance understanding of geological, environmental, and archaeological materials.
Analytical Techniques for Trace Element Detection
Trace element analysis primarily employs techniques such as Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Atomic Absorption Spectroscopy (AAS) to quantify elemental concentrations with high sensitivity and precision. Isotopic signature analysis often uses Multi-Collector ICP-MS (MC-ICP-MS) to measure isotope ratios, providing insights into geochemical sources and processes. Both methods require sample preparation protocols tailored to minimize contamination and enhance detection limits for accurate geochemical characterization.
Methods for Isotopic Signature Analysis
Isotopic signature analysis primarily employs mass spectrometry techniques such as Thermal Ionization Mass Spectrometry (TIMS) and Multi-Collector Inductively Coupled Plasma Mass Spectrometry (MC-ICP-MS) to measure precise ratios of stable or radiogenic isotopes in samples. These methods enable high-resolution differentiation of isotopic compositions, crucial for provenance studies, environmental tracing, and geological dating. Compared to trace element analysis, which uses techniques like ICP-AES or XRF for elemental concentration, isotopic signature methods provide nuanced insights into source, age, and process history through isotopic fractionation patterns.
Comparative Accuracy and Sensitivity
Trace element analysis offers high sensitivity in detecting minor elemental variations within samples, but its accuracy can be influenced by matrix effects and instrumental calibration. Isotopic signature analysis provides superior accuracy due to its ability to measure isotope ratios precisely, often with greater reproducibility across different laboratories. Comparative studies show isotopic methods excel in distinguishing source origins, whereas trace element analysis is valuable for detailed compositional profiling despite slightly lower sensitivity to subtle differences.
Sample Preparation: Best Practices and Challenges
Sample preparation for trace element analysis demands meticulous contamination control and homogenization to ensure accurate quantification of elements at ultra-trace levels, often involving acid digestion and clean lab techniques. Isotopic signature analysis requires preservation of isotopic ratios, necessitating chemical separation methods such as ion exchange chromatography that prevent isotopic fractionation and matrix effects. Both methods face challenges in minimizing sample loss, avoiding contamination, and achieving reproducibility, with best practices including the use of high-purity reagents, clean-room environments, and rigorous quality control protocols.
Applications in Environmental and Geological Studies
Trace element analysis provides critical data on the concentration of metals and minerals in soils and water, enabling identification of pollution sources and mineral deposits. Isotopic signature techniques offer precise age dating and origin tracing of rocks, sediments, and contaminants, enhancing studies of geological processes and environmental changes. Combining both methods improves accuracy in tracking pollutant pathways and understanding Earth's geochemical history.
Advantages and Limitations of Trace Element Analysis
Trace element analysis offers the advantage of identifying elemental concentrations in samples, enabling detailed material characterization and source attribution with relatively low cost and faster processing times compared to isotopic methods. Limitations include potential contamination, matrix effects, and lower specificity for provenance studies since trace elements often vary due to multiple environmental or geological factors. Unlike isotopic signature analysis, which provides precise, unique fingerprints for tracing origins, trace element analysis may lack the resolution needed for distinguishing highly similar sources.
Strengths and Weaknesses of Isotopic Signature Methods
Isotopic signature methods excel in providing precise source attribution and chronological information through stable or radiogenic isotope ratios, making them invaluable in fields like geology, archaeology, and environmental science. Their strength lies in distinguishing between similar materials and tracing biogeochemical cycles with high specificity, but they require sophisticated instrumentation such as mass spectrometers and extensive sample preparation, increasing cost and complexity. Limitations include potential isotopic fractionation during sample handling and environmental processes that can obscure original signals, as well as lower sensitivity to trace contaminants compared to elemental analyses.
Choosing the Right Technique: Factors and Recommendations
Selecting between trace element analysis and isotopic signature methods depends on the sample type, research objectives, and required resolution. Trace element analysis excels in detecting elemental concentrations for provenance or contamination studies, while isotopic signature offers precise insights into sources and processes through isotopic ratio variations. Consider factors like sensitivity, specificity, and cost-effectiveness to determine the optimal technique aligned with analytical goals and sample characteristics.
Trace element analysis Infographic
 libterm.com
libterm.com