Salinization occurs when excessive salts accumulate in soil, reducing its fertility and hindering crop growth. This process is often driven by improper irrigation practices and poor drainage, leading to significant agricultural challenges. Discover effective strategies to combat salinization and protect your soil's health in the rest of the article.
Table of Comparison
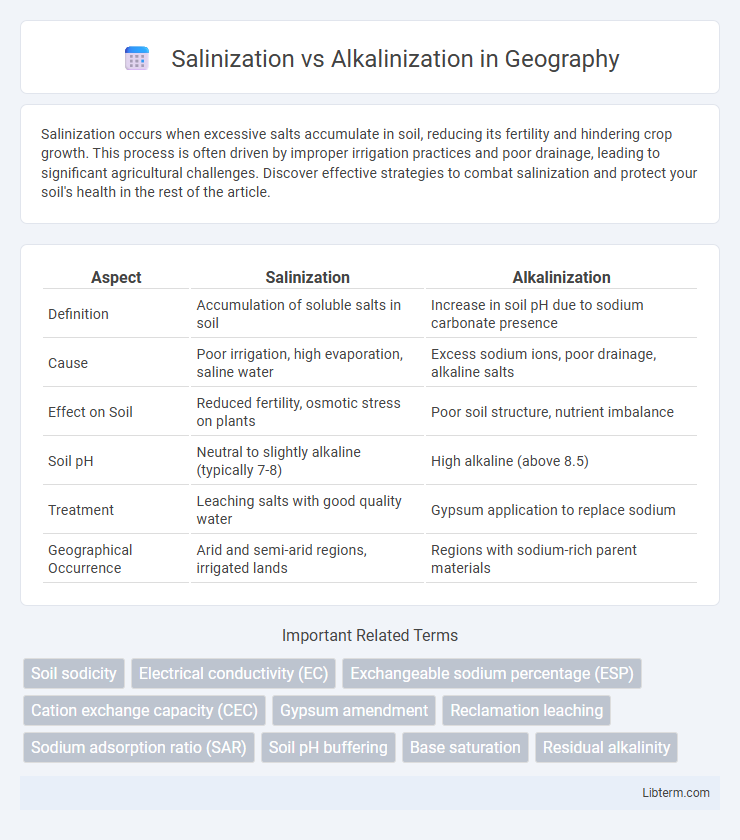
| Aspect | Salinization | Alkalinization |
|---|---|---|
| Definition | Accumulation of soluble salts in soil | Increase in soil pH due to sodium carbonate presence |
| Cause | Poor irrigation, high evaporation, saline water | Excess sodium ions, poor drainage, alkaline salts |
| Effect on Soil | Reduced fertility, osmotic stress on plants | Poor soil structure, nutrient imbalance |
| Soil pH | Neutral to slightly alkaline (typically 7-8) | High alkaline (above 8.5) |
| Treatment | Leaching salts with good quality water | Gypsum application to replace sodium |
| Geographical Occurrence | Arid and semi-arid regions, irrigated lands | Regions with sodium-rich parent materials |
Introduction to Salinization and Alkalinization
Salinization refers to the accumulation of soluble salts in soil, which impairs plant growth and reduces agricultural productivity, often resulting from improper irrigation practices or natural processes in arid regions. Alkalinization involves the increase of soil pH due to high concentrations of sodium carbonate and bicarbonate, leading to poor soil structure and nutrient deficiencies in crops. Both phenomena negatively impact soil health but differ in their chemical composition and effects on soil properties.
Defining Salinization: Causes and Processes
Salinization refers to the accumulation of soluble salts in soil or water, primarily caused by natural processes like mineral weathering and saline water intrusion, as well as human activities such as irrigation with saline water and improper drainage. This process leads to increased soil salinity, which negatively affects soil structure, nutrient availability, and crop health. Understanding salinization involves analyzing factors such as evapotranspiration, salt deposition, and groundwater rise that contribute to salt concentration in the root zone.
Understanding Alkalinization: Key Mechanisms
Alkalinization primarily occurs when soils accumulate excessive amounts of sodium carbonate or bicarbonate, leading to increased pH levels above 8.5, which disrupts soil structure and nutrient availability. Key mechanisms include the replacement of calcium by sodium on soil particles, causing dispersion and reduced permeability that impairs water infiltration. This process contrasts with salinization, where soluble salts accumulate without significantly altering pH, emphasizing the critical role of sodium speciation in soil alkalinization dynamics.
Sources of Salts Leading to Salinization
Salinization primarily results from the accumulation of soluble salts such as sodium chloride, calcium carbonate, and magnesium sulfate in soil or water, often due to natural processes like weathering of minerals, seawater intrusion, and evaporation in arid regions. Human activities including irrigation with poor-quality water, excessive use of chemical fertilizers, and inadequate drainage systems significantly contribute to salt buildup, exacerbating salinization in agricultural lands. Unlike alkalinization, which is characterized by increased soil pH due to carbonate and bicarbonate ions, salinization focuses on overall salt concentration affecting soil structure and plant growth.
Factors Contributing to Alkalinization
Alkalinization primarily results from the accumulation of sodium carbonate and bicarbonate salts in the soil, often exacerbated by poor drainage and high evaporation rates in arid and semi-arid regions. Excessive irrigation with alkaline water and inadequate leaching also contribute to elevated soil pH levels, leading to reduced nutrient availability and crop growth challenges. Soil mineralogy, particularly the presence of calcium and magnesium carbonates, plays a significant role in buffering and influencing the extent of alkalinization.
Impacts of Salinization on Soil Health
Salinization reduces soil fertility by increasing salt concentration, leading to osmotic stress that inhibits plant water uptake and nutrient absorption. High salinity disrupts soil microbial communities essential for nutrient cycling, further degrading soil health and crop productivity. This process also causes soil structure deterioration, reducing aeration and water infiltration, which exacerbates plant stress and soil erosion.
Effects of Alkalinization on Crop Production
Alkalinization in soil causes a rise in pH levels above 8.5, leading to nutrient imbalances that reduce the availability of essential elements such as iron, manganese, and phosphorus, critical for crop growth. This condition often results in poor crop yields, chlorosis, and stunted plant development due to impaired nutrient uptake. Long-term alkalinization exacerbates soil structure degradation, hindering root penetration and water absorption in crops like wheat, maize, and rice.
Comparing Salinization vs Alkalinization: Major Differences
Salinization involves the accumulation of soluble salts in soil, primarily sodium chloride and sulfate, which impairs water absorption by plants, while alkalinization refers to the increase of soil pH, often above 8.5, due to excess sodium carbonate and bicarbonate, affecting nutrient availability. Salinized soils exhibit high electrical conductivity (EC), typically exceeding 4 dS/m, whereas alkaline soils show elevated pH levels with low to moderate salinity. The impact on crop productivity differs, with salinization causing osmotic stress and ion toxicity, and alkalinization leading to nutrient imbalances and poor soil structure.
Management and Mitigation Strategies
Effective management of salinization involves leaching accumulated salts through controlled irrigation and improving soil drainage to prevent salt buildup. Mitigation of alkalinization requires the application of gypsum or sulfur amendments to neutralize high soil pH, combined with organic matter incorporation to enhance soil structure and nutrient availability. Both processes benefit from regular soil testing and adopting crop rotations with salt-tolerant and pH-resilient species to sustain soil health.
Future Perspectives and Sustainable Solutions
Future perspectives on salinization and alkalinization emphasize the integration of advanced soil sensing technologies and machine learning models to predict and mitigate soil degradation. Sustainable solutions involve adopting salt-tolerant crop varieties, efficient irrigation management, and biochar application to restore soil health while preserving agricultural productivity. Policymakers and farmers are encouraged to implement integrated water-soil management frameworks to balance economic growth with environmental conservation.
Salinization Infographic
 libterm.com
libterm.com