Toxicity refers to the degree to which a substance can cause harm to living organisms, affecting health through exposure to chemicals, pollutants, or environmental factors. Understanding toxicity levels is crucial for evaluating risks and ensuring safety in everyday products and environments. Discover how to identify toxic threats and protect Your well-being by reading the rest of this article.
Table of Comparison
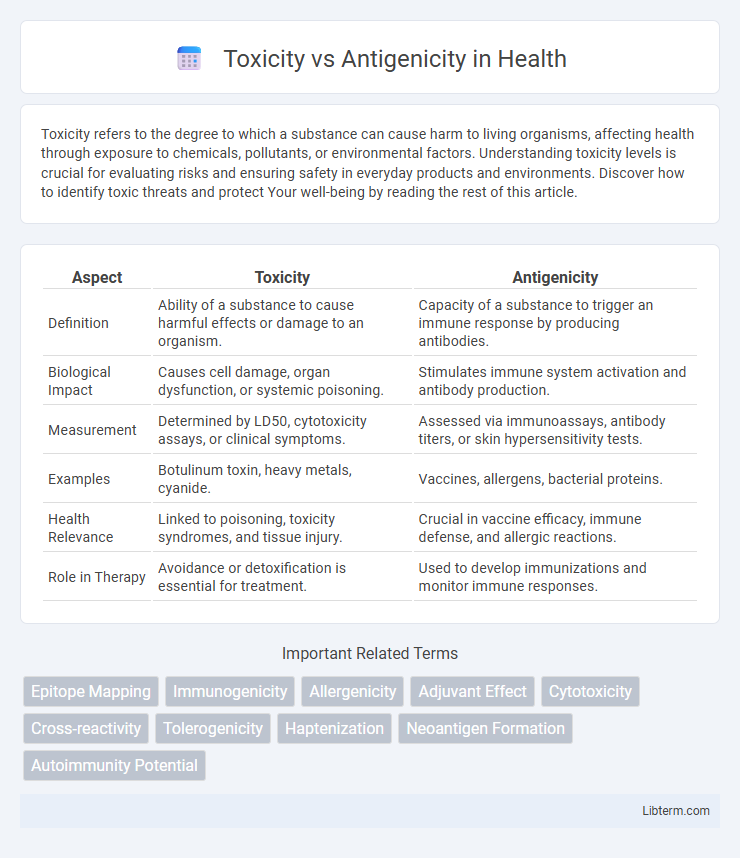
| Aspect | Toxicity | Antigenicity |
|---|---|---|
| Definition | Ability of a substance to cause harmful effects or damage to an organism. | Capacity of a substance to trigger an immune response by producing antibodies. |
| Biological Impact | Causes cell damage, organ dysfunction, or systemic poisoning. | Stimulates immune system activation and antibody production. |
| Measurement | Determined by LD50, cytotoxicity assays, or clinical symptoms. | Assessed via immunoassays, antibody titers, or skin hypersensitivity tests. |
| Examples | Botulinum toxin, heavy metals, cyanide. | Vaccines, allergens, bacterial proteins. |
| Health Relevance | Linked to poisoning, toxicity syndromes, and tissue injury. | Crucial in vaccine efficacy, immune defense, and allergic reactions. |
| Role in Therapy | Avoidance or detoxification is essential for treatment. | Used to develop immunizations and monitor immune responses. |
Understanding Toxicity and Antigenicity
Toxicity refers to the degree to which a substance can cause harmful effects to an organism, primarily through biochemical interactions that disrupt normal cellular functions. Antigenicity describes a substance's ability to bind specifically to immune receptors and elicit an immune response, particularly by stimulating the production of antibodies. Understanding toxicity involves assessing dose-response relationships and mechanisms of cellular damage, while antigenicity centers on molecular structures recognized by the immune system, such as epitopes on proteins or polysaccharides.
Key Differences Between Toxicity and Antigenicity
Toxicity refers to the degree to which a substance can cause harmful effects or damage to living organisms, while antigenicity is the ability of a substance, typically a protein or polysaccharide, to trigger an immune response by being recognized as foreign by the immune system. Toxicity primarily concerns the direct physical or biochemical damage caused by toxins, whereas antigenicity involves the identification and stimulation of immune defenses without necessarily causing harm. Key differences include toxicity's focus on harmful impact and dose-dependent effects, contrasted with antigenicity's role in immune recognition and specificity, often assessed through immune assays and antibody production.
Molecular Mechanisms of Toxicity
Toxicity arises from molecular interactions that disrupt cellular homeostasis, often by targeting critical proteins, enzymes, or membrane integrity, leading to cell damage or death. Specific molecular mechanisms include the binding of toxins to receptors or ion channels, inhibition of key metabolic pathways, and generation of reactive oxygen species causing oxidative stress. In contrast, antigenicity involves the recognition of molecules by the immune system's antibodies or T-cell receptors, triggering an immune response without necessarily causing cellular damage.
Biological Basis of Antigenicity
Antigenicity is determined by the molecular structure and composition of an antigen, specifically its ability to bind to specific receptors on immune cells and elicit an immune response. This biological basis involves the presence of epitopes or antigenic determinants, which are recognized by antibodies or T-cell receptors, driving the activation of the adaptive immune system. Toxicity, in contrast, refers to the harmful effects caused by a substance and is independent of the antigen's capacity to stimulate immunity.
Impact of Toxicity on Human Health
Toxicity refers to a substance's ability to cause harm or damage to living organisms, impacting vital organs, cellular processes, and biological functions, often leading to acute or chronic health conditions. Exposure to toxic agents can result in symptoms ranging from mild irritation to severe poisoning, organ failure, or death, depending on the toxicity level and exposure duration. Understanding the toxicological impact is crucial for risk assessment and the development of safety regulations to protect human health from hazardous chemicals and biological toxins.
Role of Antigenicity in Immune Response
Antigenicity plays a crucial role in the immune response by enabling the immune system to recognize and bind specific molecules, called antigens, on pathogens or foreign substances. This recognition triggers the activation of immune cells, including B cells and T cells, leading to the production of antibodies and the destruction or neutralization of the invading agent. Unlike toxicity, which refers to harmful effects caused by a substance, antigenicity is specifically related to the immune system's ability to detect and respond to antigens, facilitating targeted immune defense.
Factors Influencing Toxicity and Antigenicity
Factors influencing toxicity and antigenicity include the molecular structure, dose, and route of exposure, which determine the intensity and type of immune response. Chemical composition and protein conformation significantly affect antigenicity by altering the recognition by immune receptors, while toxicity is strongly influenced by the presence of reactive groups and metabolic activation. Environmental conditions, such as pH and temperature, can modify both toxicity and antigenicity by impacting molecular stability and interaction with biological targets.
Methods for Assessing Toxicity
Methods for assessing toxicity include in vitro assays such as cytotoxicity tests using mammalian cell cultures, which measure cell viability and membrane integrity after exposure to substances. In vivo studies involve administering potential toxins to animal models to observe physiological, biochemical, and histopathological changes, providing comprehensive toxicity profiles. Advanced techniques like high-throughput screening and computational modeling predict toxic effects by analyzing molecular interactions and dose-response relationships, enhancing early identification of harmful agents.
Evaluating Antigenicity in Laboratory Settings
Evaluating antigenicity in laboratory settings involves measuring the immune response triggered by a substance, primarily using techniques such as enzyme-linked immunosorbent assay (ELISA), flow cytometry, and radioimmunoassay to quantify antibody binding and T-cell activation. Toxicity assessment distinguishes harmful effects on cells or organisms, often through cytotoxicity assays like MTT or LDH release, helping to separate adverse reactions from antigen-specific immune responses. Precise antigenicity evaluation is crucial in vaccine development and biopharmaceutical safety, ensuring the immune system recognition without inducing toxic effects.
Therapeutic Implications of Toxicity and Antigenicity
Toxicity and antigenicity significantly impact the safety and efficacy of therapeutic agents, influencing drug development and patient outcomes. High toxicity necessitates dose adjustments or alternative treatments to minimize harmful side effects, while elevated antigenicity can trigger immune responses leading to reduced drug efficacy or adverse reactions. Optimizing therapeutic design involves balancing these factors to enhance treatment tolerability and immunogenic safety in clinical applications.
Toxicity Infographic
 libterm.com
libterm.com