Hypertonic solutions have a higher concentration of solutes compared to the inside of a cell, causing water to move out and resulting in cell shrinkage. This process is essential in medical treatments, such as managing cerebral edema or dehydration correction. Explore the article to understand how hypertonic solutions affect your body and their practical applications.
Table of Comparison
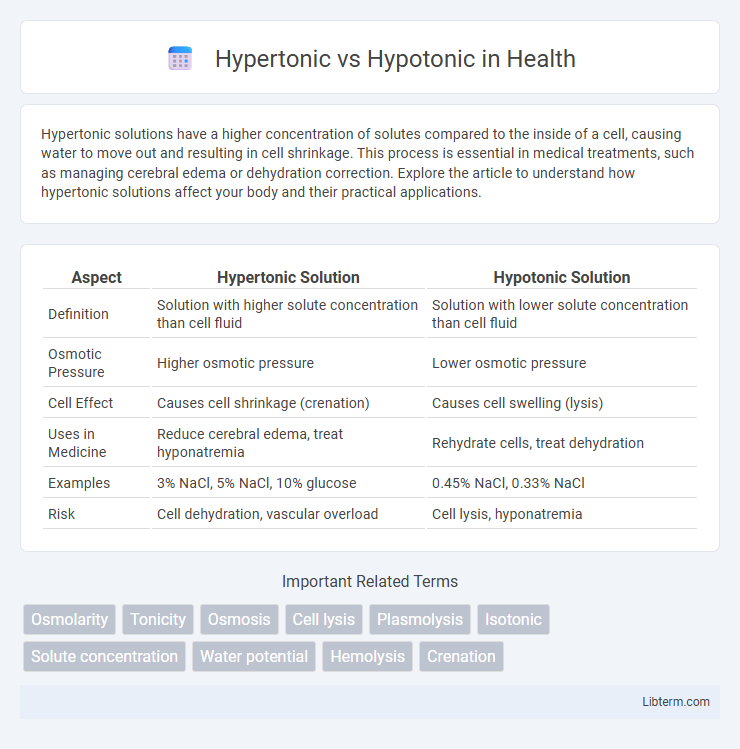
| Aspect | Hypertonic Solution | Hypotonic Solution |
|---|---|---|
| Definition | Solution with higher solute concentration than cell fluid | Solution with lower solute concentration than cell fluid |
| Osmotic Pressure | Higher osmotic pressure | Lower osmotic pressure |
| Cell Effect | Causes cell shrinkage (crenation) | Causes cell swelling (lysis) |
| Uses in Medicine | Reduce cerebral edema, treat hyponatremia | Rehydrate cells, treat dehydration |
| Examples | 3% NaCl, 5% NaCl, 10% glucose | 0.45% NaCl, 0.33% NaCl |
| Risk | Cell dehydration, vascular overload | Cell lysis, hyponatremia |
Introduction to Tonicity: Hypertonic vs Hypotonic
Tonicity refers to the relative concentration of solutes dissolved in solution which determines the direction and extent of water movement across a semipermeable membrane. Hypertonic solutions contain a higher concentration of solutes compared to the cell's interior, causing water to move out of the cell and resulting in cell shrinkage. Hypotonic solutions have a lower solute concentration than the cell's cytoplasm, leading to water influx and potential cell swelling or lysis.
Understanding Cell Membrane and Osmosis
Hypertonic solutions have higher solute concentrations than the cell's interior, causing water to move out of the cell through osmosis and resulting in cell shrinkage. Hypotonic solutions contain lower solute concentrations, prompting water to flow into the cell, which can lead to swelling or bursting. The cell membrane's selective permeability regulates this osmotic balance, maintaining cellular homeostasis by controlling solute and water movement.
What Is a Hypertonic Solution?
A hypertonic solution contains a higher concentration of solutes compared to the inside of a cell, causing water to move out of the cell by osmosis. This results in cell shrinkage or crenation as water exits to balance the solute gradient. Hypertonic solutions are commonly used in medical treatments like reducing cerebral edema or as intravenous fluids to draw fluid out of swollen tissues.
What Is a Hypotonic Solution?
A hypotonic solution has a lower concentration of solutes compared to the inside of a cell, causing water to move into the cell by osmosis. This influx of water can lead to cell swelling and potential lysis if the imbalance is severe. Hypotonic solutions are commonly used in medical treatments to rehydrate cells by restoring fluid balance.
Key Differences Between Hypertonic and Hypotonic Solutions
Hypertonic solutions have a higher solute concentration compared to the inside of a cell, causing water to move out of the cell and resulting in cell shrinkage. Hypotonic solutions contain a lower solute concentration than the cell's interior, leading to water moving into the cell and potential cell swelling or bursting. The osmotic pressure difference between these solutions determines the direction of water movement across cell membranes.
Effects on Animal Cells: Hypertonic vs Hypotonic
Hypertonic solutions cause animal cells to shrink as water exits the cell due to osmotic pressure, leading to crenation and impaired cellular functions. Hypotonic solutions result in water influx, causing animal cells to swell and potentially burst, a process known as lysis. Maintaining isotonic conditions is critical to preserve cell integrity and optimal physiological function.
Effects on Plant Cells: Hypertonic vs Hypotonic
Hypertonic solutions cause plant cells to lose water, leading to plasmolysis where the cell membrane pulls away from the cell wall, resulting in wilting and loss of turgor pressure. Hypotonic solutions lead to water entering the plant cells, increasing turgor pressure and keeping cells firm and rigid, which is essential for maintaining structural integrity. The balance between hypertonic and hypotonic environments is crucial for optimal plant cell function and overall plant health.
Real-Life Applications in Medicine and Biology
Hypertonic solutions, characterized by higher solute concentration than the surrounding cells, are used in medicine for dehydrating swollen brain tissue and treating hyponatremia by drawing water out of cells. Hypotonic solutions, with lower solute concentration, are applied to rehydrate cells in cases like diabetic ketoacidosis or hypernatremia, promoting fluid movement into cells. Both solutions are critical in intravenous therapy, influencing cellular fluid balance and ensuring effective patient electrolyte management.
Common Misconceptions About Tonicity
Common misconceptions about tonicity include the belief that hypertonic solutions always cause cells to shrink and hypotonic solutions always cause cells to swell, ignoring the influence of cell type and membrane permeability. Many assume isotonic solutions have no effect on cells, but isotonicity merely means no net water movement; subtle cellular responses can still occur. Understanding the precise solute concentration gradients and osmotic balance is crucial for accurately predicting cellular behavior in hypertonic versus hypotonic environments.
Summary Table: Hypertonic vs Hypotonic Solutions
Hypertonic solutions have a higher solute concentration than the intracellular fluid, causing cells to shrink as water moves out. Hypotonic solutions possess a lower solute concentration compared to the cell's interior, leading to cell swelling due to water influx. The summary table highlights these osmotic differences, emphasizing their impact on cell volume and fluid balance.
Hypertonic Infographic
 libterm.com
libterm.com