Antioncogenes, also known as tumor suppressor genes, play a critical role in regulating cell growth and preventing cancer by inhibiting uncontrolled cell proliferation. Mutations or inactivation of these genes can lead to the development of various cancers, making their study crucial for understanding tumorigenesis. Explore the full article to learn how your antioncogenes safeguard your cells and what happens when they fail.
Table of Comparison
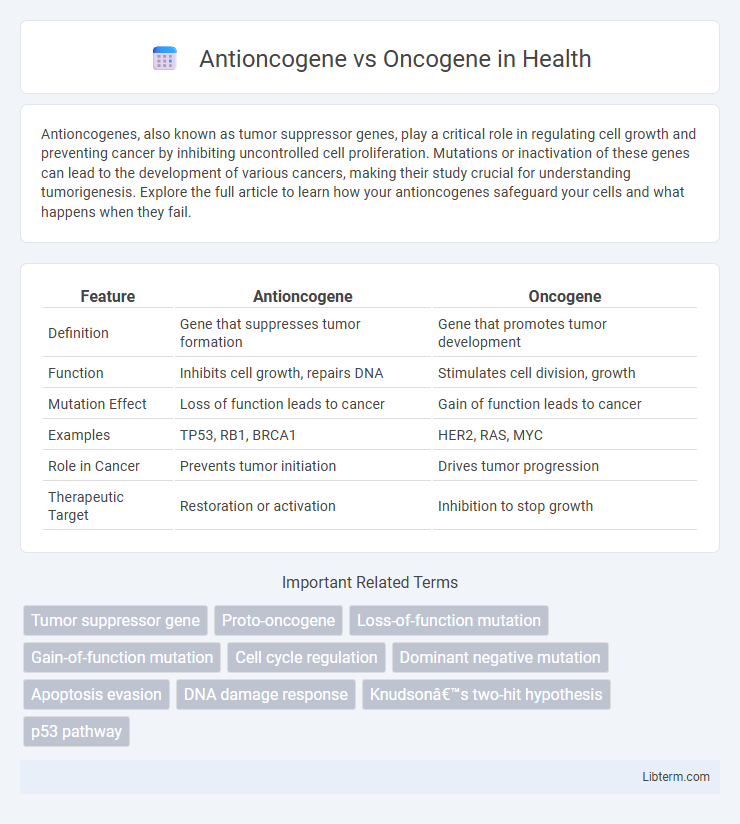
| Feature | Antioncogene | Oncogene |
|---|---|---|
| Definition | Gene that suppresses tumor formation | Gene that promotes tumor development |
| Function | Inhibits cell growth, repairs DNA | Stimulates cell division, growth |
| Mutation Effect | Loss of function leads to cancer | Gain of function leads to cancer |
| Examples | TP53, RB1, BRCA1 | HER2, RAS, MYC |
| Role in Cancer | Prevents tumor initiation | Drives tumor progression |
| Therapeutic Target | Restoration or activation | Inhibition to stop growth |
Introduction to Antioncogenes and Oncogenes
Antioncogenes, also known as tumor suppressor genes, regulate cell growth and prevent tumor formation by inhibiting uncontrolled cell division. Oncogenes are mutated or overexpressed forms of proto-oncogenes that promote cancer development by stimulating excessive cell proliferation. The delicate balance between antioncogenes and oncogenes is crucial for maintaining normal cellular function and preventing carcinogenesis.
Defining Antioncogenes: The Tumor Suppressors
Antioncogenes, also known as tumor suppressor genes, play a crucial role in regulating cell growth and preventing uncontrolled proliferation that leads to cancer. These genes encode proteins responsible for repairing DNA damage, promoting apoptosis, and inhibiting cell division when cellular abnormalities are detected. Mutations or inactivation of tumor suppressor genes, such as TP53 and RB1, disrupt these protective mechanisms and contribute to oncogenesis by allowing cells to evade normal growth controls.
Understanding Oncogenes: Drivers of Cancer
Oncogenes are mutated or overexpressed versions of normal genes called proto-oncogenes that promote uncontrolled cell division and tumor growth. Unlike tumor suppressor genes (antioncogenes), which inhibit cell proliferation and induce apoptosis, oncogenes drive cancer progression by encoding proteins that stimulate pathways such as MAPK and PI3K/AKT. Key examples of oncogenes include RAS, MYC, and HER2, whose activation results in disrupted cell cycle regulation and malignancy.
Key Molecular Differences: Antioncogenes vs Oncogenes
Antioncogenes, also known as tumor suppressor genes, encode proteins that regulate cell growth and promote apoptosis, preventing uncontrolled proliferation, whereas oncogenes arise from mutated proto-oncogenes that drive continuous cell division and survival. Key molecular differences include loss-of-function mutations in antioncogenes leading to reduced tumor suppression, contrasted with gain-of-function mutations in oncogenes resulting in constitutive activation of growth-promoting signaling pathways. Examples of major antioncogenes are TP53 and RB1, while prominent oncogenes include RAS and MYC, each playing distinct roles in carcinogenesis through opposing regulatory mechanisms.
Mechanisms of Action: Inhibition vs Promotion of Tumor Growth
Antioncogenes function by inhibiting tumor growth through mechanisms such as cell cycle arrest, DNA repair facilitation, and induction of apoptosis, thereby maintaining genomic stability. Oncogenes promote tumor growth by encoding proteins that drive uncontrolled cell proliferation, inhibit apoptotic pathways, and enhance angiogenesis, leading to malignancy. The balance between antioncogene activity and oncogene signaling is crucial in regulating cellular homeostasis and preventing tumor development.
Common Examples of Antioncogenes and Oncogenes
Common examples of antioncogenes include TP53, RB1, and BRCA1, which function as tumor suppressors by regulating cell cycle and promoting apoptosis. Oncogenes such as HER2, MYC, and RAS are frequently mutated or overexpressed in various cancers, leading to uncontrolled cell proliferation. Mutations in antioncogenes typically result in loss of function, whereas oncogene activation causes gain of function contributing to tumor development.
Mutations and Their Impact: Loss vs Gain of Function
Mutations in oncogenes typically result in a gain of function, causing the gene to become overactive and drive uncontrolled cell proliferation, which contributes to cancer development. In contrast, mutations in antioncogenes (tumor suppressor genes) often lead to a loss of function, disabling their role in regulating cell growth and apoptosis, thereby allowing abnormal cell survival. The balance between oncogene activation and antioncogene inactivation is crucial for maintaining normal cellular homeostasis and preventing tumorigenesis.
Diagnostic Approaches: Identifying Genetic Alterations
Diagnostic approaches for identifying genetic alterations in oncogenes and antioncogenes primarily involve next-generation sequencing (NGS), fluorescence in situ hybridization (FISH), and polymerase chain reaction (PCR) techniques. NGS enables comprehensive genomic profiling to detect point mutations, amplifications, and deletions in oncogenes like KRAS and antioncogenes such as TP53. FISH is employed to visualize chromosomal translocations and gene amplifications, while PCR allows for sensitive amplification and detection of specific genetic variants, facilitating personalized cancer diagnosis and targeted therapy decisions.
Therapeutic Strategies: Targeting Oncogenes and Restoring Antioncogenes
Therapeutic strategies targeting oncogenes often involve small molecule inhibitors or monoclonal antibodies designed to block the aberrant signaling pathways driven by oncogenic mutations, such as those in KRAS, EGFR, or BRAF. Restoring antioncogenes, like TP53 or RB1, utilizes gene therapy, epigenetic drugs, or protein stabilization approaches to reinstate their tumor suppressor functions that regulate cell cycle and apoptosis. Combining oncogene inhibition with antioncogene restoration holds promise for synergistic cancer treatments that address both uncontrolled proliferation and loss of growth suppression.
Future Perspectives in Cancer Genetics and Treatment
Future perspectives in cancer genetics emphasize the interplay between antioncogenes, also known as tumor suppressor genes, and oncogenes in developing targeted therapies. Advances in CRISPR gene editing and personalized medicine hold promise for reactivating mutated antioncogenes or inhibiting oncogene expression, potentially halting tumor progression more effectively. Integrating multi-omics data and artificial intelligence is expected to enhance the precision of treatments tailored to genetic profiles, improving patient outcomes in oncology.
Antioncogene Infographic
 libterm.com
libterm.com