Optically active substances have the ability to rotate the plane of polarized light, a property crucial in fields like chemistry and pharmacology for identifying and characterizing chiral molecules. This rotation occurs because these substances contain asymmetric molecules that interact uniquely with polarized light. Explore the article to understand how optical activity applies to your study or research.
Table of Comparison
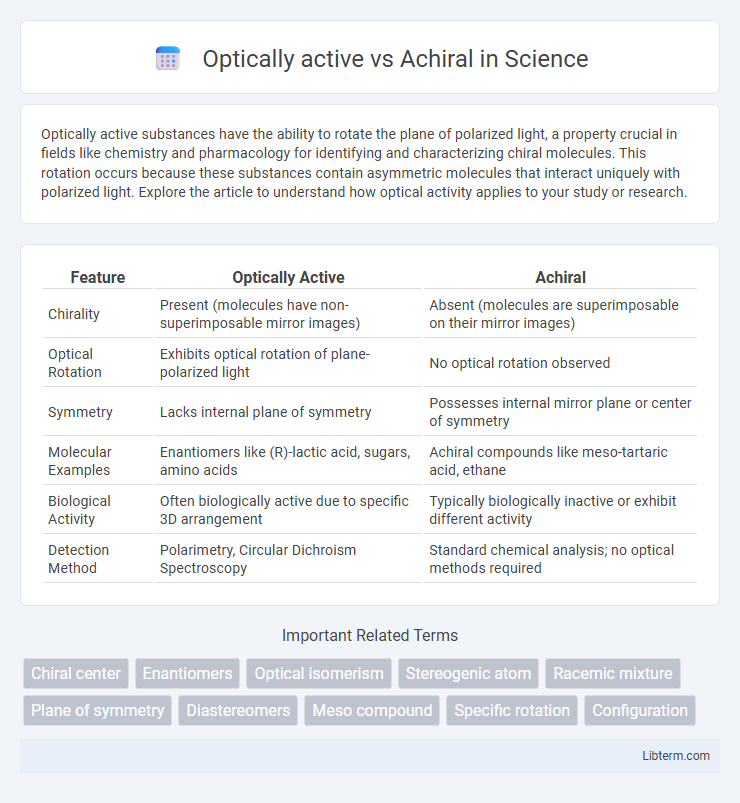
| Feature | Optically Active | Achiral |
|---|---|---|
| Chirality | Present (molecules have non-superimposable mirror images) | Absent (molecules are superimposable on their mirror images) |
| Optical Rotation | Exhibits optical rotation of plane-polarized light | No optical rotation observed |
| Symmetry | Lacks internal plane of symmetry | Possesses internal mirror plane or center of symmetry |
| Molecular Examples | Enantiomers like (R)-lactic acid, sugars, amino acids | Achiral compounds like meso-tartaric acid, ethane |
| Biological Activity | Often biologically active due to specific 3D arrangement | Typically biologically inactive or exhibit different activity |
| Detection Method | Polarimetry, Circular Dichroism Spectroscopy | Standard chemical analysis; no optical methods required |
Introduction to Optical Activity and Chirality
Optical activity refers to the ability of certain molecules to rotate plane-polarized light, a property exhibited by chiral compounds that lack an internal plane of symmetry. Chirality arises when a molecule has non-superimposable mirror images, known as enantiomers, which are optically active and differ in the direction and degree of light rotation. Achiral molecules, possessing symmetry elements such as planes or centers of inversion, do not rotate plane-polarized light and thus are optically inactive.
Defining Optically Active Compounds
Optically active compounds are chemical substances that rotate the plane of polarized light due to their chiral molecular structures, which lack an internal plane of symmetry. These compounds, typically enantiomers, possess at least one stereogenic center causing non-superimposable mirror images, leading to optical rotation measurable by polarimetry. In contrast, achiral compounds do not exhibit optical activity because they have symmetric structures that prevent interaction with polarized light in an optically active manner.
Understanding Achiral Molecules
Achiral molecules lack a chiral center and do not exhibit optical activity, meaning they cannot rotate plane-polarized light. These molecules possess superimposable mirror images, resulting in identical physical and chemical properties for their enantiomers. Understanding achiral molecules is crucial in stereochemistry as they contrast with optically active compounds that have non-superimposable mirror images and rotate polarized light.
Key Differences Between Optically Active and Achiral Substances
Optically active substances possess chiral molecules that rotate plane-polarized light, a key characteristic absent in achiral substances which do not exhibit optical rotation due to their symmetrical structure. The presence of enantiomers in optically active compounds leads to non-superimposable mirror images, whereas achiral substances have superimposable mirror images resulting in no optical activity. Optical activity is a direct indicator of molecular chirality, making it fundamental in distinguishing chiral compounds from achiral ones in stereochemistry.
Molecular Structure and Symmetry Considerations
Optically active molecules possess chiral centers, typically carbon atoms bonded to four different substituents, resulting in non-superimposable mirror images and the ability to rotate plane-polarized light. Achiral molecules lack such asymmetric centers or have internal planes of symmetry, causing them to be superimposable on their mirror images and optically inactive. The presence or absence of molecular symmetry elements like mirror planes or inversion centers fundamentally determines the optical activity of a compound.
How Optical Activity is Measured
Optical activity is measured using a polarimeter, which quantifies the rotation of plane-polarized light as it passes through an optically active substance, typically a chiral compound. The degree of rotation, called specific rotation, depends on factors such as concentration, path length, temperature, and wavelength of light used. Achiral substances do not exhibit optical rotation since their molecular structures are superimposable on their mirror images, resulting in no measurable rotation of plane-polarized light.
Real-World Examples of Optically Active vs Achiral Compounds
Lactic acid is a classic example of an optically active compound due to its chiral center, causing it to rotate plane-polarized light. In contrast, benzene represents an achiral compound, possessing a symmetrical planar structure with no optical activity. These distinctions highlight the importance of molecular symmetry and chirality in the behavior of optically active versus achiral substances in real-world chemical applications.
Importance of Chirality in Chemistry and Biology
Optically active compounds exhibit chirality, meaning they have non-superimposable mirror images that rotate plane-polarized light, a critical property influencing molecular interactions in chemistry and biology. Achiral molecules lack this asymmetric structure and do not affect polarized light, often resulting in different biological activity compared to chiral counterparts. The importance of chirality lies in its impact on drug efficacy, enzyme specificity, and biochemical pathways, where only one enantiomer may exhibit the desired biological response, emphasizing the necessity of chirality control in pharmaceutical design and biochemistry.
Applications of Optical Activity in Industry and Research
Optically active compounds, which rotate plane-polarized light, are pivotal in pharmaceuticals for enantiomerically pure drug synthesis, ensuring higher efficacy and reduced side effects. In the chemical industry, optical activity facilitates quality control and stereochemical analysis during production of agrochemicals and flavors. Research applications exploit chiral discrimination in spectroscopy and chromatography, advancing asymmetric synthesis and understanding biomolecular interactions.
Summary: Implications in Stereochemistry
Optically active compounds possess chirality, causing them to rotate plane-polarized light, a key factor in stereochemical analysis and drug design. Achiral molecules lack this optical activity, as their mirror images are superimposable, influencing properties like reactivity and interaction with biological targets. Understanding the distinction between optically active and achiral substances is crucial for predicting stereochemical behavior and developing enantiomerically pure pharmaceuticals.
Optically active Infographic
 libterm.com
libterm.com