Diathermal materials allow heat to pass through them easily, facilitating thermal conduction without significant resistance. Understanding the properties of diathermal substances is crucial for applications in thermodynamics and heat transfer engineering. Explore the rest of this article to learn how diathermal principles impact your daily life and modern technology.
Table of Comparison
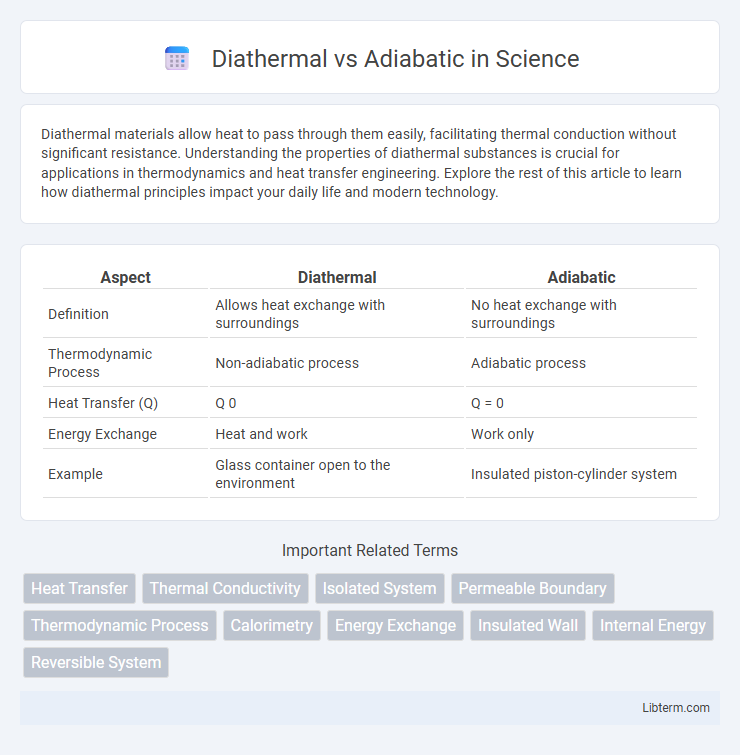
| Aspect | Diathermal | Adiabatic |
|---|---|---|
| Definition | Allows heat exchange with surroundings | No heat exchange with surroundings |
| Thermodynamic Process | Non-adiabatic process | Adiabatic process |
| Heat Transfer (Q) | Q 0 | Q = 0 |
| Energy Exchange | Heat and work | Work only |
| Example | Glass container open to the environment | Insulated piston-cylinder system |
Introduction to Diathermal and Adiabatic Processes
Diathermal processes allow heat transfer between a system and its surroundings, enabling temperature changes through thermal energy exchange. Adiabatic processes occur without heat exchange, maintaining constant internal energy while work is done on or by the system. Understanding these distinctions is crucial in thermodynamics for analyzing energy transfer and system behavior under different thermal conditions.
Definition of Diathermal Systems
Diathermal systems allow the transfer of heat between the system and its surroundings, enabling thermal energy exchange without restriction. These systems are characterized by boundaries that permit heat flow, facilitating temperature equilibration with the environment. In contrast, adiabatic systems prevent heat transfer, maintaining thermal isolation throughout the process.
Definition of Adiabatic Systems
Adiabatic systems are thermodynamic systems that do not exchange heat with their surroundings, meaning all energy transfer occurs only through work. These systems maintain constant entropy during an ideal adiabatic process, reflecting no heat transfer across the system boundary. The defining characteristic of adiabatic systems is their perfect thermal insulation, preventing heat flow while allowing changes in internal energy through mechanical work.
Key Differences Between Diathermal and Adiabatic
Diathermal processes allow heat exchange between a system and its surroundings, enabling thermal equilibrium changes, whereas adiabatic processes prevent any heat transfer, maintaining internal energy changes solely due to work done. The key difference lies in thermal insulation: diathermal boundaries are heat-permeable, while adiabatic boundaries are thermally insulating. This distinction critically influences thermodynamic cycles and energy transfer analyses in physical and engineering systems.
Thermodynamic Principles Involved
Diathermal processes allow heat exchange between a system and its surroundings, adhering to the first law of thermodynamics where energy transfer occurs as heat or work. Adiabatic processes prohibit heat transfer, isolating the system thermally and causing internal energy changes solely through work interactions, often described by the relation PV^g = constant for ideal gases. The distinction fundamentally impacts entropy changes, with diathermal processes potentially increasing entropy, whereas ideal adiabatic processes are isentropic, maintaining constant entropy in reversible conditions.
Real-World Examples of Diathermal Processes
Diathermal processes occur when a system exchanges heat with its surroundings, such as boiling water in an open pot where heat flows from the stove to the liquid. Another example is refrigeration, where heat is transferred from the interior to the external environment through the appliance walls. These real-world examples highlight the importance of conductive and convective heat transfer in diathermal systems.
Real-World Examples of Adiabatic Processes
Real-world examples of adiabatic processes include the rapid compression of air in a bicycle pump and the expansion of air in a hot air balloon. During these processes, no heat is exchanged with the surroundings, causing temperature changes solely due to pressure variations. Adiabatic cooling in rising air masses contributes to cloud formation and weather patterns.
Applications in Engineering and Science
Diathermal processes, allowing heat exchange, are critical in thermodynamic systems like heat engines and refrigeration cycles, where energy transfer optimizes efficiency and performance. Adiabatic processes, characterized by no heat transfer, are essential in compressors, turbines, and atmospheric dynamics to model rapid pressure and temperature changes without heat loss. Understanding these processes enables precise control in chemical reactors, HVAC systems, and aerodynamic simulations, driving advancements in energy conservation and system design.
Advantages and Limitations of Each System
Diathermal systems enable heat exchange with their surroundings, allowing temperature regulation and increased process control, but they may suffer from energy losses and reduced efficiency. Adiabatic systems prevent heat transfer, ensuring energy conservation and ideal insulation, which benefits processes requiring constant internal energy, though they limit temperature control and can lead to internal temperature fluctuations. Understanding these trade-offs is crucial for optimizing thermodynamic system design based on specific application requirements.
Conclusion and Summary
Diathermal processes involve heat exchange with the surroundings, allowing energy transfer through thermal conduction, whereas adiabatic processes occur without heat transfer, maintaining system energy internally. Understanding the distinction is crucial in thermodynamics for predicting system behavior, especially in engines, atmospheric science, and refrigeration cycles. Accurate modeling of these processes enhances efficiency and performance in heat engines and thermal management systems.
Diathermal Infographic
 libterm.com
libterm.com