Cooperativity enhances the efficiency of molecular interactions by enabling multiple binding sites to work together, often resulting in a more than additive effect on biological function. This phenomenon is crucial in processes such as oxygen transport by hemoglobin, where the binding of one oxygen molecule increases the affinity for subsequent molecules. Discover how understanding cooperativity can impact your knowledge of biochemistry and molecular biology in the rest of this article.
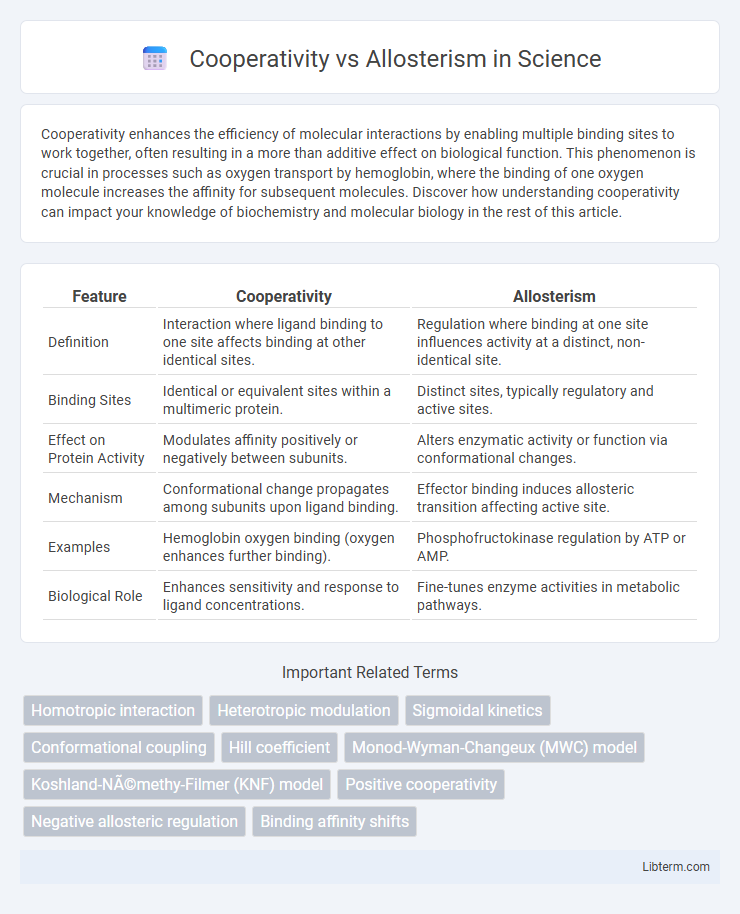
Table of Comparison
| Feature | Cooperativity | Allosterism |
|---|---|---|
| Definition | Interaction where ligand binding to one site affects binding at other identical sites. | Regulation where binding at one site influences activity at a distinct, non-identical site. |
| Binding Sites | Identical or equivalent sites within a multimeric protein. | Distinct sites, typically regulatory and active sites. |
| Effect on Protein Activity | Modulates affinity positively or negatively between subunits. | Alters enzymatic activity or function via conformational changes. |
| Mechanism | Conformational change propagates among subunits upon ligand binding. | Effector binding induces allosteric transition affecting active site. |
| Examples | Hemoglobin oxygen binding (oxygen enhances further binding). | Phosphofructokinase regulation by ATP or AMP. |
| Biological Role | Enhances sensitivity and response to ligand concentrations. | Fine-tunes enzyme activities in metabolic pathways. |
Introduction to Cooperativity and Allosterism
Cooperativity refers to the interaction between binding sites on a multi-subunit protein, where the binding of a ligand to one site affects the affinity at other sites, exemplified by hemoglobin's oxygen binding. Allosterism involves the regulation of a protein's activity through the binding of an effector molecule at a site other than the active site, leading to conformational changes that modulate function. Both cooperativity and allosterism are key mechanisms in biochemical regulation, enhancing the efficiency and specificity of molecular interactions.
Defining Cooperativity: Mechanisms and Examples
Cooperativity refers to the phenomenon where the binding of a ligand to one site on a multi-subunit protein affects the binding affinity at other sites, often enhancing or inhibiting subsequent ligand interactions. Mechanisms include positive cooperativity, exemplified by hemoglobin's oxygen binding, and negative cooperativity observed in enzymes like aspartate transcarbamoylase. This interdependent binding modulates protein function and regulatory processes essential for cellular signaling and metabolism.
Understanding Allosterism: Basic Principles
Allosterism involves the regulation of a protein's activity through the binding of an effector molecule at a site other than the active site, causing conformational changes that alter function. This mechanism enables proteins to respond dynamically to cellular signals, often resulting in either activation or inhibition of enzymatic activity. Understanding allosterism requires analyzing the structural shifts and communication pathways within the protein that facilitate these regulatory effects.
Structural Basis of Cooperativity in Proteins
The structural basis of cooperativity in proteins is primarily linked to conformational changes that occur upon ligand binding, influencing subsequent ligand affinity at other sites. Hemoglobin serves as a classical example, where its quaternary structure shifts between the tense (T) and relaxed (R) states, modulating oxygen binding through cooperative interactions between subunits. These structural transitions often involve alterations in protein subunit interfaces and dynamic rearrangements, facilitating enhanced communication and affinity changes across active sites.
Types of Allosteric Regulation
Allosteric regulation involves reversible changes in enzyme activity through effector binding at sites distinct from the active site, categorized into homotropic and heterotropic types. Homotropic allosteric regulation occurs when the substrate itself acts as the effector, often enhancing enzyme activity through positive cooperativity. Heterotropic allosteric regulation involves distinct molecules as effectors, which can either activate or inhibit the enzyme, modulating metabolic pathways with high specificity.
Key Differences Between Cooperativity and Allosterism
Cooperativity refers to the multiple binding sites on a protein that influence each other's affinity for a ligand, typically seen in hemoglobin where oxygen binding increases affinity at other sites. Allosterism involves the regulation of protein activity through conformational changes induced by effector molecules binding at a site distinct from the active site, affecting protein function beyond ligand binding affinity. The key difference lies in cooperativity describing ligand binding interactions between identical sites, whereas allosterism encompasses broader regulatory mechanisms involving distinct effector sites.
Biological Significance of Cooperativity
Cooperativity enhances enzyme efficiency by allowing substrate binding at one active site to increase affinity at other sites, crucial in oxygen transport by hemoglobin. This biological mechanism ensures rapid and regulated response to changing substrate concentrations, optimizing metabolic pathways and cellular processes. Cooperativity's role in signal transduction and regulation highlights its significance in maintaining homeostasis and cellular communication.
Allosterism in Drug Discovery and Design
Allosterism plays a crucial role in drug discovery by targeting allosteric sites on proteins, offering selectivity and fewer side effects compared to orthosteric ligands. Allosteric modulators can fine-tune protein function, enabling the design of drugs that precisely regulate receptor activity and overcome issues like drug resistance. Exploiting allosteric mechanisms accelerates the development of innovative therapeutics across various diseases, including neurological disorders and cancer.
Experimental Approaches to Study Cooperativity and Allosterism
Experimental approaches to study cooperativity and allosterism primarily involve techniques like enzyme kinetics assays, isothermal titration calorimetry (ITC), and nuclear magnetic resonance (NMR) spectroscopy. Enzyme kinetics provides quantitative data on substrate binding rates and affinity changes, revealing cooperative behavior. ITC measures heat changes during ligand binding, offering thermodynamic insights, while NMR elucidates conformational changes associated with allosteric regulation in proteins.
Future Perspectives in Cooperativity and Allosteric Research
Future perspectives in cooperativity and allosteric research emphasize the integration of advanced computational models with high-resolution structural techniques to unravel dynamic protein conformations. Emerging approaches using cryo-electron microscopy paired with machine learning algorithms enable precise mapping of allosteric sites and cooperative interactions, facilitating novel drug design strategies targeting complex signaling pathways. Enhanced understanding of spatiotemporal regulation in cellular environments is expected to drive breakthroughs in therapeutics and synthetic biology applications.
Cooperativity Infographic
 libterm.com
libterm.com