Electrodes are critical components in various electrical and electronic devices, serving as conductors that allow current to enter or exit a medium. They are commonly used in batteries, welding, electrolysis, and medical equipment, with materials selected based on conductivity, durability, and reactivity. Discover how the choice and function of electrodes impact your devices by exploring the rest of this article.
Table of Comparison
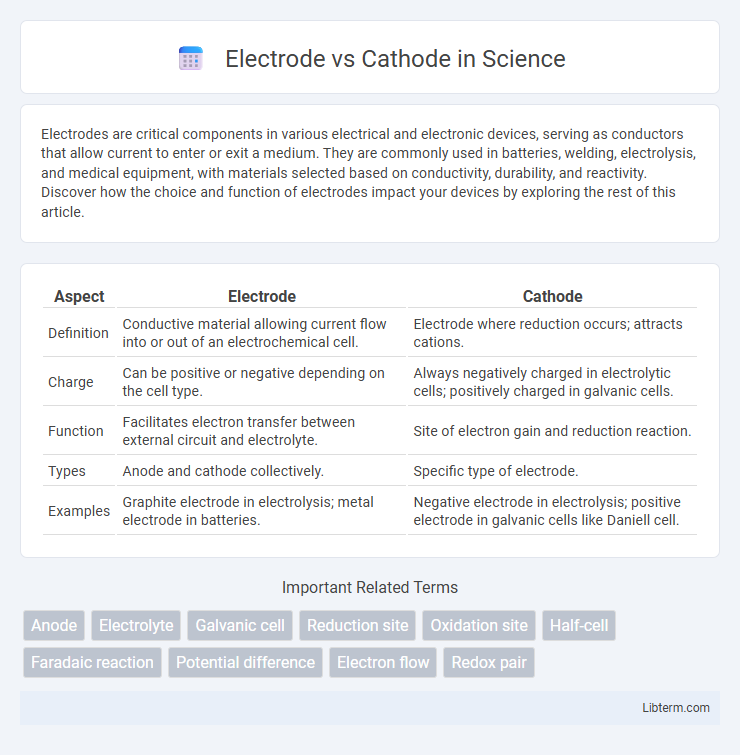
| Aspect | Electrode | Cathode |
|---|---|---|
| Definition | Conductive material allowing current flow into or out of an electrochemical cell. | Electrode where reduction occurs; attracts cations. |
| Charge | Can be positive or negative depending on the cell type. | Always negatively charged in electrolytic cells; positively charged in galvanic cells. |
| Function | Facilitates electron transfer between external circuit and electrolyte. | Site of electron gain and reduction reaction. |
| Types | Anode and cathode collectively. | Specific type of electrode. |
| Examples | Graphite electrode in electrolysis; metal electrode in batteries. | Negative electrode in electrolysis; positive electrode in galvanic cells like Daniell cell. |
Introduction to Electrodes and Cathodes
Electrodes are conductive materials that serve as the interface for electron transfer in electrochemical cells, facilitating oxidation or reduction reactions. Cathodes specifically refer to the electrode where reduction occurs, attracting cations during the electrochemical process. Understanding the distinction between electrodes and cathodes is essential for optimizing battery performance, electrolysis, and corrosion prevention.
Definition of an Electrode
An electrode is a conductor through which electric current enters or leaves a nonmetallic medium, such as an electrolyte, semiconductor, or vacuum. It serves as a critical interface in electrochemical cells, facilitating oxidation and reduction reactions. Unlike the cathode, which specifically refers to the electrode where reduction occurs, the term electrode encompasses both anode and cathode as general components in electrical circuits.
Understanding the Cathode
The cathode is the electrode where reduction reactions occur, meaning it gains electrons during electrochemical processes. In a galvanic cell, the cathode is the positive electrode because it attracts cations and facilitates their gain of electrons. Understanding the cathode's role is crucial for optimizing battery efficiency and designing electrochemical sensors.
Key Differences Between Electrode and Cathode
Electrodes are the conductive materials through which electric current enters or leaves a non-metallic medium, while cathodes specifically refer to the electrode where reduction occurs, and electrons flow into the device. The key difference lies in their roles: the cathode is always the site of electron gain in electrochemical cells, whereas electrodes can serve as either anodes or cathodes depending on the direction of current and type of cell. Understanding this distinction is crucial for applications ranging from batteries to electrolysis and semiconductor devices.
Roles of Electrode and Cathode in Electrochemical Cells
In electrochemical cells, the electrode serves as a conductor for electrons, facilitating the redox reactions essential for energy conversion. The cathode specifically acts as the site of reduction, where electrons are gained during the electrochemical process. Both the electrode and cathode play crucial roles in determining the cell's overall efficiency and functionality.
Types of Electrodes: Anode vs. Cathode
Anodes and cathodes are two primary types of electrodes distinguished by their roles in electrochemical cells; the anode is the electrode where oxidation occurs, releasing electrons, while the cathode is the electrode where reduction takes place, gaining electrons. In galvanic cells, the anode is negative and the cathode is positive, whereas in electrolytic cells, the anode is positive and the cathode is negative. Understanding the anode-cathode distinction is crucial for applications in batteries, electroplating, and corrosion protection.
Applications of Electrodes in Various Industries
Electrodes serve as essential components in diverse industries such as electronics, welding, and electrochemical cells by facilitating electron transfer and conduction. Cathodes, specifically, act as the site of reduction reactions in batteries, electroplating, and fuel cells, driving energy storage and conversion processes. Industrial applications rely on tailored electrode materials like graphite, metal alloys, and conductive polymers to optimize performance in sensors, corrosion protection, and energy devices.
Importance of Cathodes in Modern Technology
Cathodes serve as the essential electrodes where reduction reactions occur, playing a crucial role in batteries, electrolysis, and electronic devices. Their ability to efficiently accept electrons enables the functionality of lithium-ion batteries, OLED displays, and cathode ray tubes. Advanced cathode materials enhance energy density, cycle life, and performance in modern technology, driving innovations in renewable energy storage and consumer electronics.
Common Materials Used for Electrodes and Cathodes
Common materials used for electrodes include graphite, platinum, gold, and carbon due to their high conductivity and chemical stability. Cathodes in batteries often consist of lithium cobalt oxide, manganese dioxide, or nickel-cadmium compounds, which facilitate efficient electron transfer and energy storage. The choice of electrode and cathode materials significantly influences the performance and lifespan of electrochemical cells.
Summary: Choosing Between Electrode and Cathode
Choosing between electrode and cathode depends on the specific electrochemical application, as the electrode is a general term for any conductor through which current enters or leaves a medium, while the cathode specifically refers to the electrode where reduction occurs. In batteries, cells, or electrolysis, the cathode attracts cations and undergoes reduction, making its role critical in defining current flow and chemical reactions. Understanding the functional difference ensures proper device design, optimizing efficiency and performance in energy storage, electroplating, or sensor technologies.
Electrode Infographic
 libterm.com
libterm.com