Diffusion is the process by which molecules spread from an area of higher concentration to one of lower concentration, facilitating essential biological and chemical exchanges. This natural movement plays a critical role in various systems, including cellular respiration and air purification. Explore the article to understand how diffusion impacts your everyday life and its practical applications.
Table of Comparison
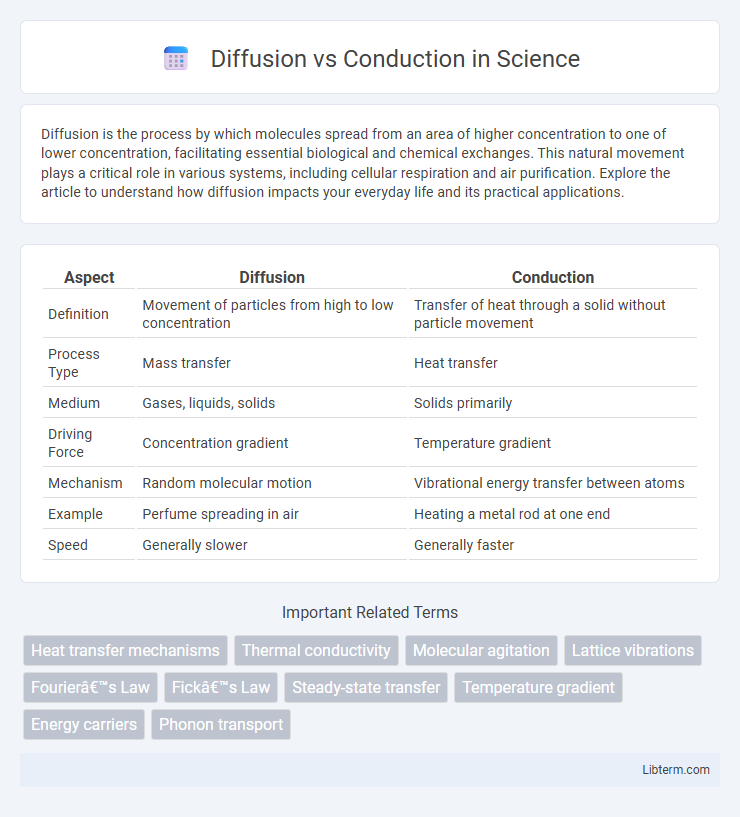
| Aspect | Diffusion | Conduction |
|---|---|---|
| Definition | Movement of particles from high to low concentration | Transfer of heat through a solid without particle movement |
| Process Type | Mass transfer | Heat transfer |
| Medium | Gases, liquids, solids | Solids primarily |
| Driving Force | Concentration gradient | Temperature gradient |
| Mechanism | Random molecular motion | Vibrational energy transfer between atoms |
| Example | Perfume spreading in air | Heating a metal rod at one end |
| Speed | Generally slower | Generally faster |
Introduction to Diffusion and Conduction
Diffusion is the process where particles move from an area of higher concentration to an area of lower concentration, driven by random molecular motion, and is essential in mixing gases and liquids. Conduction involves the transfer of heat through a material without the movement of the material itself, relying on the vibration and collision of molecules in solids. Both diffusion and conduction are fundamental mechanisms of energy and mass transfer in physical and biological systems.
Defining Diffusion: Concepts and Mechanisms
Diffusion is the process of particles spreading from areas of higher concentration to lower concentration due to random molecular motion, driven by thermal energy. It occurs without the need for external energy input, relying on the natural movement of atoms or molecules to equalize concentration gradients. Key mechanisms include Brownian motion and concentration gradient forces, essential in gas exchange, nutrient transport, and chemical reactions.
Understanding Conduction: Principles and Processes
Conduction is the transfer of heat through a solid material due to the vibration and collision of adjacent atoms or molecules, governed by Fourier's law which states that the heat flux is proportional to the negative gradient of temperature. Materials with high thermal conductivity, such as metals like copper and aluminum, facilitate faster conduction by allowing free electrons to carry thermal energy efficiently. Understanding conduction involves analyzing thermal conductivity coefficients, temperature gradients, and the molecular lattice structure that dictates how heat energy propagates without any bulk movement of the material itself.
Key Differences Between Diffusion and Conduction
Diffusion is the process where particles move from an area of higher concentration to lower concentration due to random molecular motion, while conduction involves heat transfer through direct contact between molecules in a solid. Diffusion occurs primarily in gases and liquids, whereas conduction is the dominant heat transfer mechanism in solids. The rate of diffusion depends on concentration gradients and temperature, whereas conduction depends on temperature gradients and the thermal conductivity of the material.
Examples of Diffusion in Everyday Life
Diffusion occurs when molecules move from an area of higher concentration to lower concentration, such as the spreading of perfume scent across a room or the gradual mixing of cream into coffee. In contrast, conduction involves heat transfer through direct contact, like a metal spoon warming up in hot soup. Everyday examples of diffusion include oxygen entering the bloodstream through lung alveoli and sugar dissolving uniformly in tea without stirring.
Real-World Applications of Conduction
Conduction plays a critical role in everyday applications such as cooking, where heat transfers from stove burners to pots and pans, ensuring efficient temperature control. Building insulation materials leverage conduction principles to regulate indoor temperatures by reducing heat flow through walls and windows. In electronics, heat sinks utilize conduction to dissipate heat generated by components, preventing overheating and maintaining device performance.
Factors Affecting Diffusion and Conduction Rates
Diffusion rates are primarily influenced by temperature, concentration gradient, and the medium's properties, such as porosity and viscosity, which determine the ease with which particles move. Conduction rates depend on the thermal conductivity of the material, cross-sectional area, and the temperature difference across the material, with metals typically exhibiting higher conduction rates due to free electron movement. Both diffusion and conduction are accelerated by increased temperature, but diffusion relies more heavily on molecular motion while conduction depends on atomic or electron interactions within solids.
Diffusion and Conduction in Biological Systems
Diffusion in biological systems is the passive movement of molecules from regions of higher concentration to lower concentration, essential for processes such as oxygen transport across cell membranes and nutrient exchange in tissues. Conduction, primarily referring to the transfer of electrical signals in neurons, enables rapid communication through the propagation of action potentials along axons. Both mechanisms are critical for maintaining homeostasis, with diffusion facilitating molecular distribution and conduction supporting cellular signaling and coordination.
Materials and Environments: Impact on Transport Mechanisms
Materials with high thermal conductivity, such as metals, enhance heat transfer through conduction by facilitating rapid molecular vibrations and electron movement. In contrast, diffusion in gases and liquids depends on molecular concentration gradients and is significantly influenced by environmental factors like temperature, pressure, and medium viscosity. Porous or heterogeneous materials exhibit complex transport behaviors where both diffusion and conduction mechanisms interplay based on structural properties and environmental conditions.
Diffusion vs Conduction: Summary and Comparative Insights
Diffusion and conduction are fundamental processes of energy and mass transfer, where diffusion involves the movement of particles from high to low concentration driven by concentration gradients, and conduction refers to heat transfer through a solid without the movement of the material itself. Diffusion is characterized by molecular motion and is prominent in gases and liquids, while conduction is dominant in solids due to lattice vibrations and free electron movements. Comparing these mechanisms highlights diffusion's role in mass transport and conduction's importance in thermal energy transfer, both governed by their respective laws: Fick's law for diffusion and Fourier's law for conduction.
Diffusion Infographic
 libterm.com
libterm.com