Ionic is a powerful open-source framework for building cross-platform mobile applications using web technologies like HTML, CSS, and JavaScript. Its extensive library of pre-designed UI components and seamless integration with Angular, React, and Vue enable developers to create high-performance apps with native-like experiences. Discover how Ionic can transform your mobile development process by reading the full article.
Table of Comparison
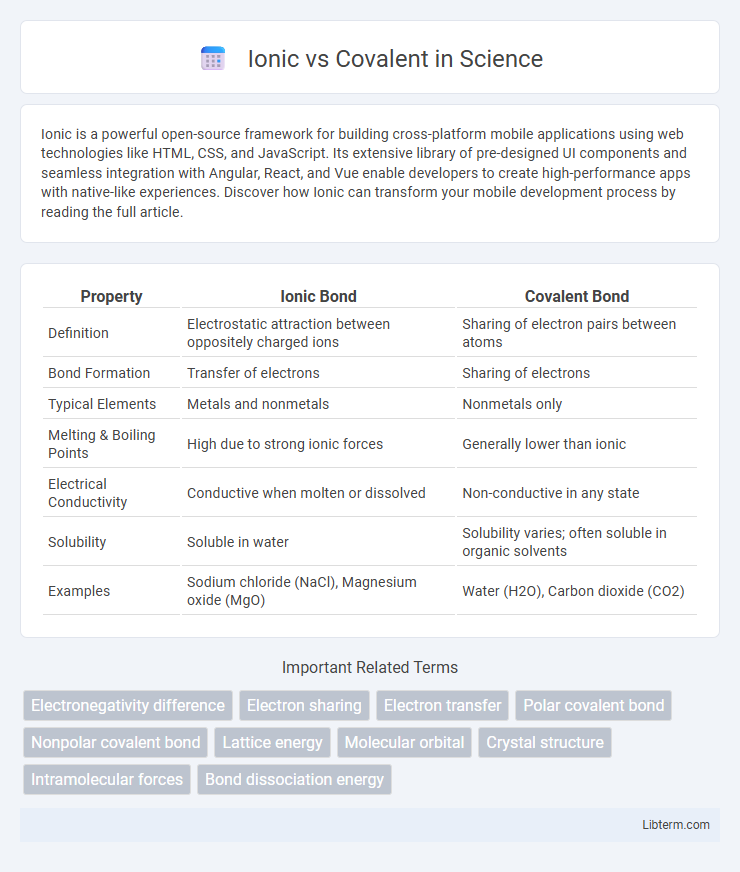
| Property | Ionic Bond | Covalent Bond |
|---|---|---|
| Definition | Electrostatic attraction between oppositely charged ions | Sharing of electron pairs between atoms |
| Bond Formation | Transfer of electrons | Sharing of electrons |
| Typical Elements | Metals and nonmetals | Nonmetals only |
| Melting & Boiling Points | High due to strong ionic forces | Generally lower than ionic |
| Electrical Conductivity | Conductive when molten or dissolved | Non-conductive in any state |
| Solubility | Soluble in water | Solubility varies; often soluble in organic solvents |
| Examples | Sodium chloride (NaCl), Magnesium oxide (MgO) | Water (H2O), Carbon dioxide (CO2) |
Introduction to Ionic and Covalent Bonds
Ionic bonds form through the transfer of electrons from a metal to a non-metal, resulting in positively charged cations and negatively charged anions that attract each other due to electrostatic forces. Covalent bonds occur when two non-metals share electron pairs to achieve stable electron configurations, creating molecules with specific geometric shapes. Understanding electron transfer in ionic bonding and electron sharing in covalent bonding is fundamental to chemistry and the behavior of compounds.
Definition of Ionic Bonds
Ionic bonds form through the complete transfer of electrons from a metal atom to a non-metal atom, resulting in positively charged cations and negatively charged anions that attract each other due to electrostatic forces. This type of bond creates crystalline structures with high melting and boiling points, commonly found in compounds like sodium chloride (NaCl). Ionic bonds differ from covalent bonds, where electrons are shared rather than transferred between atoms.
Definition of Covalent Bonds
Covalent bonds form when atoms share one or more pairs of electrons to achieve a full outer shell, creating a stable molecule. These bonds typically occur between nonmetal atoms with similar electronegativities, resulting in electron density concentrated between the bonded nuclei. Unlike ionic bonds, which involve electron transfer and electrostatic attraction, covalent bonding emphasizes electron sharing and molecular orbital overlap.
Key Differences Between Ionic and Covalent Bonds
Ionic bonds form through the transfer of electrons between atoms, resulting in positively and negatively charged ions that create a strong electrostatic attraction, typically between metals and nonmetals. Covalent bonds involve the sharing of electron pairs between atoms, usually nonmetals, enabling the formation of molecules with shared electron densities and directional bonding. The difference in electronegativity values--greater than 1.7 for ionic and less than 1.7 for covalent bonds--is a critical factor in determining the bond type and its physical properties such as melting point, conductivity, and solubility.
Types of Compounds Formed
Ionic compounds form when metals transfer electrons to nonmetals, resulting in positively charged cations and negatively charged anions that arrange into crystalline lattices with high melting points. Covalent compounds arise from the sharing of electron pairs between nonmetal atoms, creating molecules with defined shapes and lower melting points compared to ionic solids. Ionic compounds typically conduct electricity when molten or dissolved, whereas covalent compounds usually do not conduct electricity in any state.
Physical Properties Comparison
Ionic compounds typically exhibit high melting and boiling points due to strong electrostatic forces between ions, resulting in solid, brittle structures at room temperature. Covalent compounds generally have lower melting and boiling points with variable states of matter, as their atoms share electrons and form molecules held by weaker intermolecular forces. Ionic compounds conduct electricity when molten or dissolved in water, whereas covalent compounds usually do not conduct electricity in any state.
Electrical Conductivity
Ionic compounds exhibit high electrical conductivity when molten or dissolved in water due to the mobility of their charged ions, whereas in solid form, their ions are fixed in a lattice, preventing conductivity. Covalent compounds generally have poor electrical conductivity because they consist of neutral molecules with localized electrons, lacking free charge carriers. Some covalent network solids like graphite are exceptions, conducting electricity through delocalized electrons within their planar structure.
Examples of Ionic and Covalent Compounds
Sodium chloride (NaCl) is a classic example of an ionic compound formed by the transfer of electrons between sodium and chlorine atoms, resulting in a strong electrostatic attraction. Water (H2O) exemplifies a covalent compound where hydrogen and oxygen atoms share electrons, creating distinct polar covalent bonds. Other notable ionic compounds include magnesium oxide (MgO) and calcium chloride (CaCl2), while common covalent compounds consist of carbon dioxide (CO2) and methane (CH4).
Formation Mechanisms
Ionic bonds form through the electrostatic attraction between positively charged cations and negatively charged anions, typically resulting from the complete transfer of electrons from a metal to a non-metal. Covalent bonds arise from the sharing of electron pairs between atoms, usually non-metals, to achieve stable electron configurations. The formation mechanism of ionic bonds emphasizes electron transfer and electrostatic forces, whereas covalent bonds rely on electron sharing and orbital overlap.
Real-World Applications
Ionic bonds dominate in high-melting-point compounds like sodium chloride, crucial for industrial salt production and food preservation. Covalent bonds form the backbone of organic molecules, enabling the creation of complex pharmaceuticals and polymers essential for medical and manufacturing industries. Both bond types influence material properties, guiding the development of electronics, coatings, and sustainable energy solutions.
Ionic Infographic
 libterm.com
libterm.com