Glassy surfaces offer a sleek, modern look while providing durability and ease of maintenance in various applications, from architecture to automotive design. Their transparency and reflective qualities enhance natural light and aesthetic appeal in any environment. Discover how glassy materials can transform your space by exploring the full article.
Table of Comparison
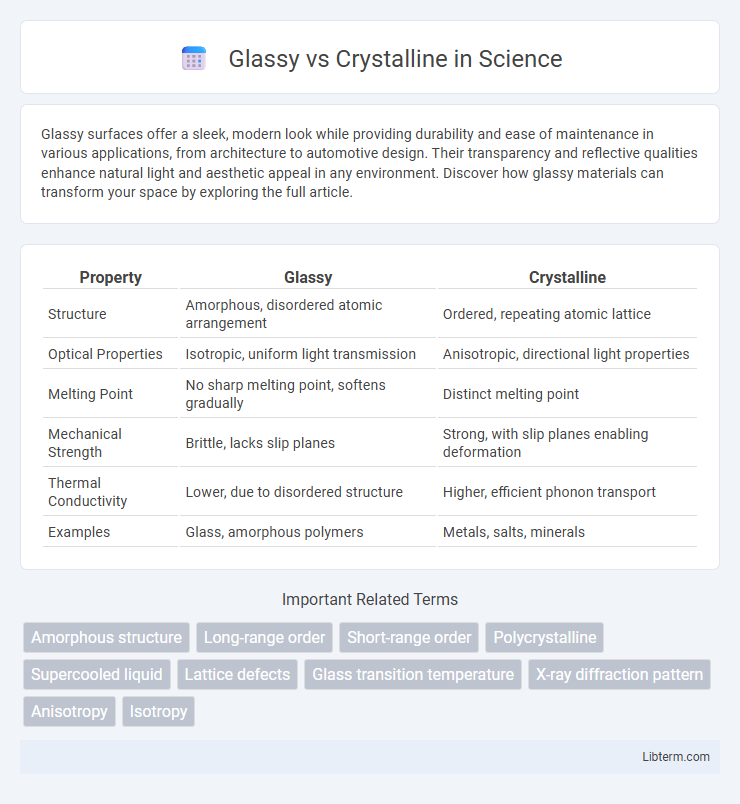
| Property | Glassy | Crystalline |
|---|---|---|
| Structure | Amorphous, disordered atomic arrangement | Ordered, repeating atomic lattice |
| Optical Properties | Isotropic, uniform light transmission | Anisotropic, directional light properties |
| Melting Point | No sharp melting point, softens gradually | Distinct melting point |
| Mechanical Strength | Brittle, lacks slip planes | Strong, with slip planes enabling deformation |
| Thermal Conductivity | Lower, due to disordered structure | Higher, efficient phonon transport |
| Examples | Glass, amorphous polymers | Metals, salts, minerals |
Introduction to Glassy and Crystalline Materials
Glassy materials exhibit a disordered atomic structure lacking long-range periodicity, resulting in isotropic physical properties and transparency in many cases. Crystalline materials possess a well-ordered, repeating lattice structure that defines their anisotropic mechanical, thermal, and electrical characteristics. Understanding the fundamental differences between glassy and crystalline states is crucial for tailoring materials in applications such as optics, semiconductors, and structural components.
Defining Glassy Structures
Glassy structures lack the long-range order found in crystalline materials, characterized by a disordered atomic arrangement that mimics the liquid state frozen in place. Unlike crystalline solids with periodic lattice patterns, glassy solids exhibit isotropic properties due to their random atomic distribution. This lack of crystallinity results in unique mechanical and optical behaviors essential for applications in materials science and technology.
Understanding Crystalline Structures
Crystalline structures exhibit a highly ordered arrangement of atoms repeated periodically in three dimensions, distinguishing them from amorphous or glassy solids which lack this regular pattern. The understanding of crystalline structures relies on analyzing unit cells, lattice parameters, and symmetry elements that define the crystal system, such as cubic, tetragonal, or hexagonal. Techniques like X-ray diffraction and electron microscopy are essential for characterizing these structures, revealing atomic positions and enabling prediction of physical properties including conductivity, hardness, and optical behavior.
Key Differences: Atomic Arrangement
Glassy materials exhibit an amorphous atomic arrangement with atoms randomly distributed and lacking long-range order, while crystalline materials have a highly ordered, repeating atomic structure forming a crystal lattice. This difference in atomic arrangement leads to distinct physical properties, such as glassy materials being isotropic and exhibiting no sharp melting point, whereas crystalline materials are anisotropic and melt at specific temperatures. The disordered atomic structure in glassy solids results from rapid cooling that prevents atoms from arranging into a stable crystal lattice.
Physical Properties: Glassy vs Crystalline
Glassy materials exhibit isotropic physical properties due to their disordered atomic structure, resulting in uniform mechanical strength and thermal expansion in all directions. Crystalline substances display anisotropic behavior, with physical properties such as hardness, cleavage, and thermal conductivity varying along different crystallographic axes due to their ordered lattice arrangement. Glassy solids generally have lower density and lack a sharp melting point, unlike crystalline solids which have a defined melting temperature and higher density.
Formation Processes and Conditions
Glassy materials form through rapid cooling or quenching of a liquid, preventing the atoms from arranging into a well-ordered crystalline structure, resulting in an amorphous solid. Crystalline materials develop via slow cooling or controlled solidification, allowing atoms to align into a repeating, long-range ordered lattice. Temperature, cooling rate, and environmental conditions significantly influence whether a material becomes glassy or crystalline during solidification.
Optical and Mechanical Characteristics
Glassy materials exhibit isotropic optical properties with uniform light transmission, while crystalline substances show anisotropic behavior due to their ordered atomic structures, resulting in birefringence and directional light refraction. Mechanically, glassy solids tend to be brittle with lower fracture toughness, lacking the slip systems found in crystalline materials that allow for plastic deformation and enhanced ductility. The contrast in atomic arrangement significantly influences their thermal expansion, hardness, and response to stress, critical for applications requiring specific optical clarity and mechanical resilience.
Common Applications in Industry
Glassy materials are extensively used in optics, electronics, and pharmaceutical industries due to their transparency, chemical resistance, and ease of shaping, making glass fibers ideal for telecommunications and display panels. Crystalline materials are predominantly employed in semiconductor manufacturing, metallurgy, and electronics, valued for their well-defined lattice structures that enable precise electrical conductivity and mechanical strength. Both material types serve critical roles in advanced manufacturing, with glassy substances enabling insulation and protection, while crystalline solids provide structural integrity and functional performance.
Pros and Cons of Each Structure
Glassy materials exhibit isotropic properties and lack long-range order, providing excellent optical clarity and corrosion resistance but often suffer from brittleness and lower thermal stability. Crystalline structures offer well-defined atomic arrangements that yield high mechanical strength, thermal conductivity, and predictable melting points, yet they may experience anisotropic behavior, grain boundary weaknesses, and susceptibility to cleavage. Understanding these trade-offs is critical for selecting materials in applications such as optics, electronics, and structural components.
Future Trends in Material Science
Future trends in material science emphasize the development of hybrid materials combining the amorphous structure of glassy solids with the ordered atomic arrangement found in crystalline materials, enhancing mechanical strength and thermal stability. Advanced computational modeling and machine learning accelerate the discovery of new glass-forming alloys and crystalline phases tailored for applications in electronics, aerospace, and energy storage. Innovations in additive manufacturing enable precise control over microstructures, fostering the fabrication of materials with customized properties that bridge the advantages of both glassy and crystalline states.
Glassy Infographic
 libterm.com
libterm.com