Electrolytes are vital for conducting electric current in batteries, while solvents dissolve these electrolytes to create a stable medium for ion transport. The choice of solvent impacts the electrolyte's conductivity, stability, and overall battery performance. Explore the article to learn how optimizing electrolytes and solvents can enhance your device's efficiency and lifespan.
Table of Comparison
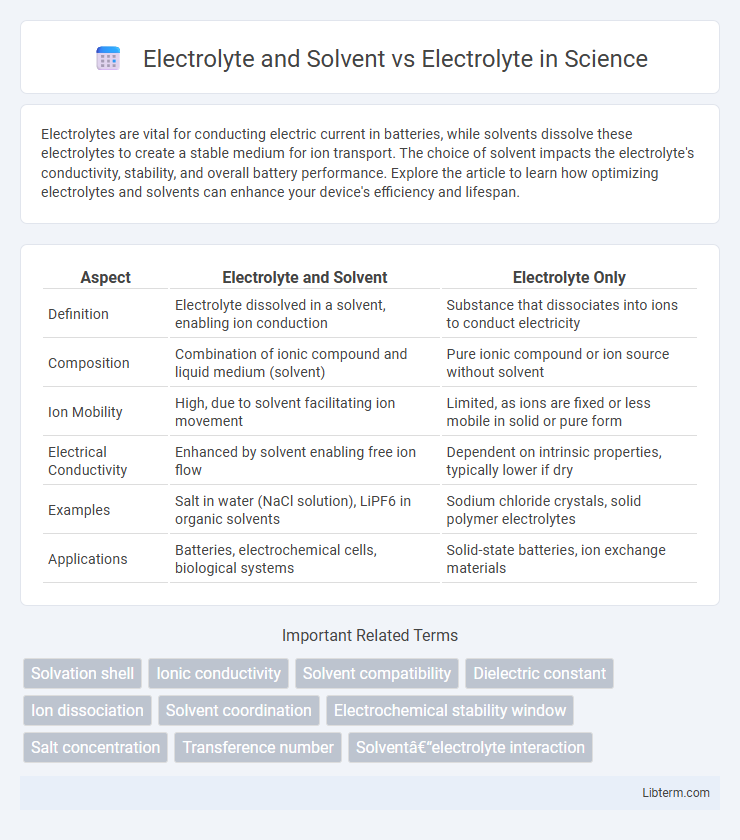
| Aspect | Electrolyte and Solvent | Electrolyte Only |
|---|---|---|
| Definition | Electrolyte dissolved in a solvent, enabling ion conduction | Substance that dissociates into ions to conduct electricity |
| Composition | Combination of ionic compound and liquid medium (solvent) | Pure ionic compound or ion source without solvent |
| Ion Mobility | High, due to solvent facilitating ion movement | Limited, as ions are fixed or less mobile in solid or pure form |
| Electrical Conductivity | Enhanced by solvent enabling free ion flow | Dependent on intrinsic properties, typically lower if dry |
| Examples | Salt in water (NaCl solution), LiPF6 in organic solvents | Sodium chloride crystals, solid polymer electrolytes |
| Applications | Batteries, electrochemical cells, biological systems | Solid-state batteries, ion exchange materials |
Understanding Electrolytes: Core Concepts
Electrolytes consist of ions dissolved in a solvent, typically water, enabling electric conductivity by facilitating ion movement. The solvent's polarity and dielectric constant significantly influence electrolyte dissociation and ion mobility, directly impacting conductivity and electrochemical performance. Understanding the interplay between electrolyte composition and solvent properties is essential for optimizing battery efficiency, corrosion resistance, and biological function.
Role of Solvents in Electrolyte Systems
Solvents in electrolyte systems play a crucial role in dissolving salts to produce ions, enhancing ionic conductivity and enabling efficient charge transport. They influence the electrolyte's viscosity, dielectric constant, and electrochemical stability, directly affecting battery performance and safety. Proper solvent selection optimizes ion solvation and mobility, ensuring stable and high-performing energy storage devices.
Electrolyte Alone: Properties and Limitations
Electrolytes alone exhibit essential properties such as high ionic conductivity and chemical stability, which are critical for efficient charge transport in batteries and fuel cells. However, their limitations include limited voltage stability windows and low solubility of certain salts, which can impede device performance. Overcoming these constraints often requires optimizing electrolyte composition or incorporating solvents to enhance ion mobility and overall electrochemical compatibility.
Electrolyte-Solvent Interactions: Scientific Overview
Electrolyte-solvent interactions critically influence ion mobility and conductivity in electrochemical systems, where solvents stabilize ions through solvation shells, enhancing dissociation and transport. Polar solvents with high dielectric constants improve electrolyte performance by reducing ion pairing, thereby increasing ionic conductivity and electrochemical stability. Understanding these interactions is essential for optimizing battery electrolytes, supercapacitors, and fuel cells, impacting energy storage efficiency and device longevity.
Comparative Analysis: Electrolyte vs. Electrolyte with Solvent
Electrolytes without solvents consist of pure ionic compounds or molten salts, offering high ionic conductivity but limited practical use due to their high melting points and viscosity. Electrolytes combined with solvents enhance ion mobility and conductivity through solvent dissociation and ion solvation, enabling efficient charge transport in applications like batteries and fuel cells. The comparative analysis reveals that solvent-based electrolytes provide greater electrochemical stability and improved ion transport dynamics, crucial for optimizing performance in energy storage technologies.
Impact on Ionic Conductivity
Electrolyte and solvent combinations significantly influence ionic conductivity by affecting ion dissociation and mobility within the solution. Electrolytes typically provide ions that conduct charge, while solvents impact the dielectric constant and viscosity, which in turn modulate ion transport efficiency. Higher dielectric solvents reduce ion pairing, enhancing conductivity, whereas low-viscosity environments facilitate faster ion movement and improved electrolyte performance.
Influence on Energy Storage Performance
Electrolyte and solvent combinations significantly influence energy storage performance by enhancing ion conductivity, stability, and electrochemical window of batteries, leading to improved capacity and cycle life. Solvents stabilize electrolyte salts and facilitate efficient ion transport, directly impacting charge-discharge rates and energy density. Optimizing electrolyte formulation with suitable solvents reduces degradation and enhances thermal stability, crucial for high-performance energy storage systems.
Safety Considerations: Pure vs. Mixed Systems
Pure electrolyte systems often present higher risks of thermal runaway and toxicity due to lack of dilution and limited heat dissipation capacity, increasing flammability and reactive hazards. Mixed electrolyte solvents improve safety by reducing volatility, enhancing thermal stability, and lowering flammability through the synergistic effects of combined components. Careful selection and optimization of solvent ratios in electrolyte mixtures significantly mitigate hazards compared to single-component systems, promoting safer battery operation and handling.
Real-World Applications of Electrolyte-Solvent Pairings
Electrolyte-solvent pairings are crucial in optimizing the performance of batteries, fuel cells, and supercapacitors by enhancing ionic conductivity and stability under operational conditions. Lithium-ion batteries, for instance, rely on specific organic solvents like ethylene carbonate paired with lithium salt electrolytes to improve charge efficiency and thermal safety. In industrial electroplating, aqueous electrolyte-solvent mixtures ensure uniform metal deposition by balancing ion transport and solvent evaporation rates.
Future Trends in Electrolyte and Solvent Research
Future trends in electrolyte and solvent research emphasize the development of high-performance, environmentally friendly materials that enhance battery safety and energy density. Innovations focus on designing solid-state electrolytes combined with solvent additives to improve ionic conductivity and thermal stability in advanced lithium-ion and sodium-ion batteries. Emerging research prioritizes sustainable, non-flammable solvents and bio-derived electrolytes to meet the growing demands of electric vehicles and grid storage applications.
Electrolyte and Solvent Infographic
 libterm.com
libterm.com