Acylation is a crucial chemical process involving the addition of an acyl group to a molecule, often improving its stability and reactivity. This reaction plays a significant role in organic synthesis and pharmaceutical development, modifying compounds to enhance their properties. Discover how acylation can impact your chemical applications and explore detailed mechanisms in the rest of this article.
Table of Comparison
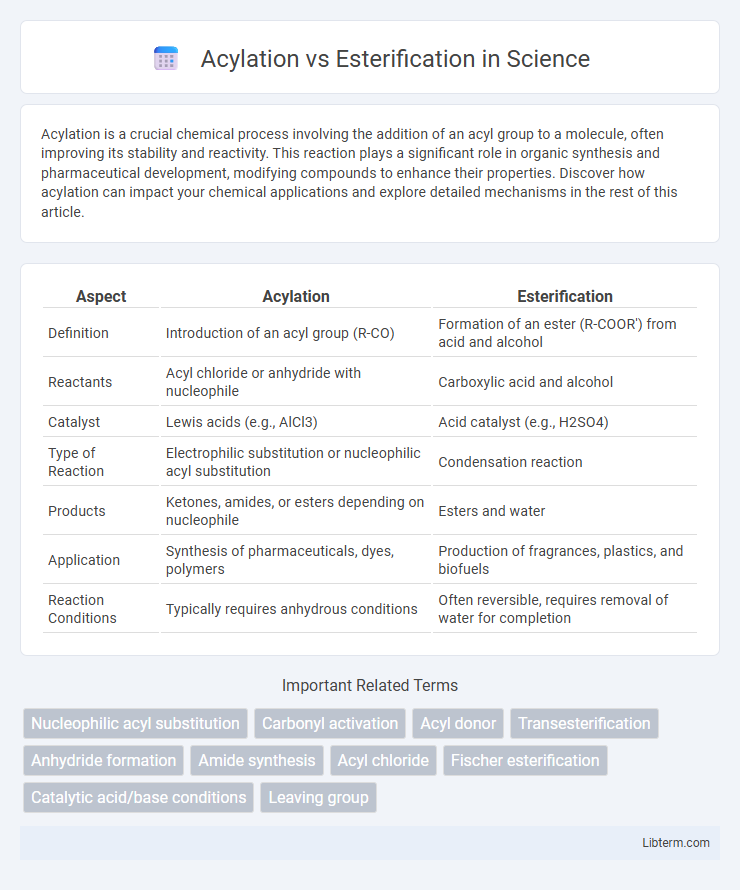
| Aspect | Acylation | Esterification |
|---|---|---|
| Definition | Introduction of an acyl group (R-CO) | Formation of an ester (R-COOR') from acid and alcohol |
| Reactants | Acyl chloride or anhydride with nucleophile | Carboxylic acid and alcohol |
| Catalyst | Lewis acids (e.g., AlCl3) | Acid catalyst (e.g., H2SO4) |
| Type of Reaction | Electrophilic substitution or nucleophilic acyl substitution | Condensation reaction |
| Products | Ketones, amides, or esters depending on nucleophile | Esters and water |
| Application | Synthesis of pharmaceuticals, dyes, polymers | Production of fragrances, plastics, and biofuels |
| Reaction Conditions | Typically requires anhydrous conditions | Often reversible, requires removal of water for completion |
Introduction to Acylation and Esterification
Acylation is a chemical reaction where an acyl group is introduced into a compound, commonly involving acyl chlorides or anhydrides as acylating agents. Esterification is a specific type of acylation that forms esters through the reaction between an acid (usually a carboxylic acid) and an alcohol, often catalyzed by acid. Both processes are fundamental in organic synthesis, enabling the modification of molecular structures to enhance chemical properties and biological activity.
Defining Acylation: Key Concepts
Acylation is a chemical reaction where an acyl group (RCO-) is introduced into a molecule, transforming the molecule by replacing a hydrogen atom or another substituent. Unlike esterification, which specifically forms esters through the reaction of an acid and an alcohol, acylation encompasses a broader range of reactions including the formation of amides, esters, and other derivatives. Key acylation reagents include acid chlorides, anhydrides, and acyl imidazoles, which facilitate the transfer of the acyl group under controlled conditions.
Understanding Esterification: Fundamental Principles
Esterification is a chemical reaction between an acid, typically a carboxylic acid, and an alcohol, resulting in the formation of an ester and water. This process involves the nucleophilic attack of the alcohol's hydroxyl group on the carbonyl carbon of the acid, facilitated by acid catalysts such as sulfuric acid to increase reaction rate and yield. Understanding esterification's fundamental principles is crucial for applications in organic synthesis, polymer production, and fragrance manufacturing, distinguishing it from acylation, which involves the introduction of an acyl group into a compound without forming water.
Types of Acylating and Esterifying Agents
Acylation typically involves acyl halides, acid anhydrides, and acyl chlorides as primary acylating agents, which efficiently introduce acyl groups into substrates. Esterification commonly utilizes carboxylic acids and alcohols, often catalyzed by strong acids like sulfuric acid or employing acid chlorides and acid anhydrides for more reactive esterifying agents. Selection of agents depends on desired reaction conditions and product specificity, with acylating agents generally exhibiting higher reactivity than conventional esterifying agents.
Mechanisms of Acylation Reactions
Acylation reactions involve the transfer of an acyl group to a nucleophile, typically proceeding through nucleophilic acyl substitution mechanisms where a tetrahedral intermediate forms. The mechanism often includes activation of the acyl compound by electrophiles such as acid chlorides, anhydrides, or activated esters, facilitating nucleophilic attack. Key steps include nucleophile addition to the carbonyl carbon, collapse of the tetrahedral intermediate, and departure of a leaving group, differentiating it from esterification which commonly involves protonation and dehydration steps.
Mechanisms of Esterification Reactions
Esterification reactions typically proceed via nucleophilic acyl substitution mechanisms, where a carboxylic acid reacts with an alcohol in the presence of an acid catalyst to form an ester and water. The process involves protonation of the carbonyl oxygen, increasing electrophilicity, followed by nucleophilic attack by the alcohol, formation of a tetrahedral intermediate, and subsequent elimination of water. In contrast, acylation involves the transfer of an acyl group from an acid derivative, such as an acyl chloride or anhydride, to a nucleophile, often proceeding through a more reactive acyl intermediate without the necessity of protonation steps seen in esterification.
Comparative Analysis: Acylation vs Esterification
Acylation and esterification are both key organic reactions involving the introduction of acyl groups but differ fundamentally in their mechanisms and applications. Acylation typically involves the transfer of an acyl group to nucleophiles such as amines or aromatic rings, often catalyzed by Lewis acids, while esterification specifically forms esters through the reaction of carboxylic acids and alcohols, frequently under acidic conditions. The reaction conditions, catalysts, and resulting functional groups distinguish acylation's broader substrate scope from esterification's targeted synthesis of esters.
Applications in Organic Synthesis
Acylation introduces an acyl group into organic molecules, widely employed in synthesizing ketones, amides, and esters critical for pharmaceuticals and polymers. Esterification, the condensation of carboxylic acids with alcohols, is crucial for producing esters used as solvents, plasticizers, and flavoring agents. Both reactions enable selective functional group transformations, enhancing molecular complexity in organic synthesis and industrial applications.
Industrial and Pharmaceutical Significance
Acylation involves introducing an acyl group into a compound, widely used in pharmaceutical synthesis for modifying drug molecules to enhance efficacy and bioavailability, while esterification converts acids and alcohols into esters, crucial in producing pharmaceuticals, polymers, and fragrances in industrial sectors. Both reactions enable the development of key intermediates and active pharmaceutical ingredients (APIs), optimizing drug properties and manufacturing efficiency. Industrially, esterification is essential in creating biodegradable plastics and solvents, whereas acylation facilitates the synthesis of complex molecules like analgesics and antibiotics.
Challenges and Advancements in Acylation and Esterification
Challenges in acylation include controlling selectivity and minimizing side reactions with sensitive functional groups, while advancements have introduced novel catalysts and green solvents to enhance reaction efficiency and sustainability. Esterification faces difficulties in shifting equilibrium toward product formation and reducing energy input, with recent progress involving enzymatic catalysis and continuous flow systems to improve yields and process scalability. Both processes benefit from innovative techniques such as microwave-assisted synthesis and tailored acid catalysts, pushing industrial applications toward greener and more cost-effective methodologies.
Acylation Infographic
 libterm.com
libterm.com