Hydrolysis is a chemical process where water molecules break down compounds into smaller components, playing a vital role in digestion, metabolism, and industrial applications. Enzymes such as amylase, lipase, and protease facilitate hydrolysis by targeting carbohydrates, fats, and proteins respectively, making complex substances easier for your body to absorb. Explore the rest of this article to understand how hydrolysis impacts biological systems and various industries in depth.
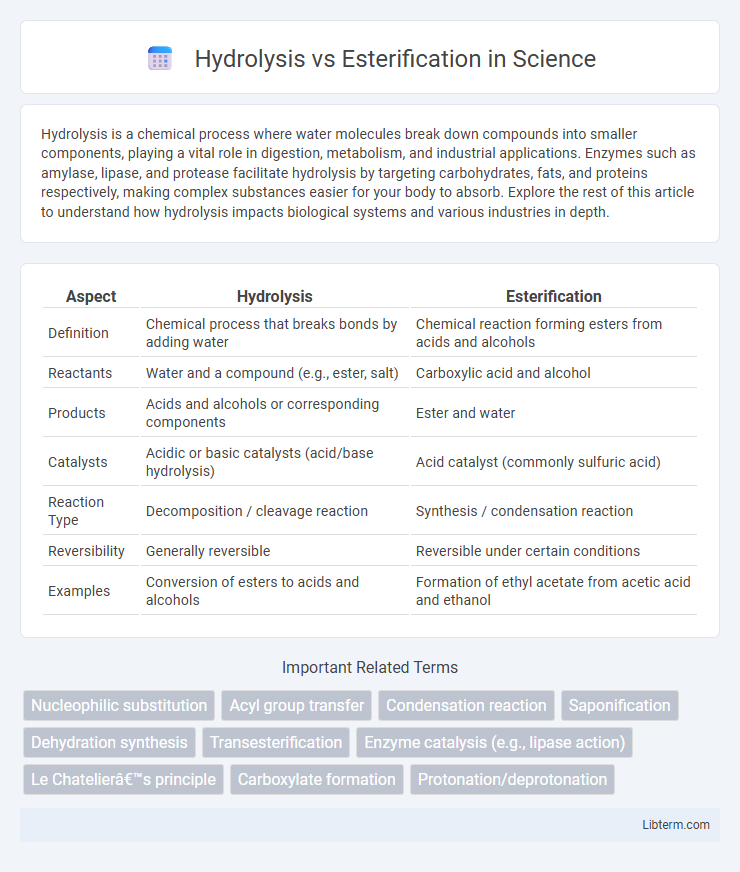
Table of Comparison
| Aspect | Hydrolysis | Esterification |
|---|---|---|
| Definition | Chemical process that breaks bonds by adding water | Chemical reaction forming esters from acids and alcohols |
| Reactants | Water and a compound (e.g., ester, salt) | Carboxylic acid and alcohol |
| Products | Acids and alcohols or corresponding components | Ester and water |
| Catalysts | Acidic or basic catalysts (acid/base hydrolysis) | Acid catalyst (commonly sulfuric acid) |
| Reaction Type | Decomposition / cleavage reaction | Synthesis / condensation reaction |
| Reversibility | Generally reversible | Reversible under certain conditions |
| Examples | Conversion of esters to acids and alcohols | Formation of ethyl acetate from acetic acid and ethanol |
Introduction to Hydrolysis and Esterification
Hydrolysis is a chemical reaction in which a water molecule breaks down a compound, often resulting in the cleavage of bonds such as ester or peptide linkages. Esterification involves the formation of an ester bond through the reaction between a carboxylic acid and an alcohol, typically catalyzed by an acid. Both processes are fundamental in organic chemistry, with hydrolysis commonly used in digestion and esterification in synthesizing fragrances and polymers.
Chemical Definitions and Basic Concepts
Hydrolysis is a chemical reaction that involves breaking down a compound by reacting it with water, often catalyzed by acids, bases, or enzymes, resulting in the cleavage of bonds such as esters into alcohols and acids. Esterification is the process of forming an ester through the reaction of an acid, typically a carboxylic acid, with an alcohol, usually catalyzed by an acid catalyst, involving the elimination of water. Both reactions are fundamental in organic chemistry, demonstrating reversible interconversion between esters and their corresponding acids and alcohols.
Mechanisms of Hydrolysis
Hydrolysis involves the cleavage of ester bonds by water molecules, typically catalyzed by acids or bases, where the nucleophilic attack of hydroxide or water on the carbonyl carbon leads to the breakdown of the ester into an alcohol and a carboxylic acid or its salt. Acid-catalyzed hydrolysis proceeds through protonation of the ester carbonyl oxygen, increasing electrophilicity and facilitating nucleophilic attack, while base-catalyzed hydrolysis (saponification) involves direct nucleophilic attack by hydroxide ions, resulting in an irreversible reaction. The key mechanistic steps include nucleophilic addition, tetrahedral intermediate formation, and elimination, differentiating hydrolysis from esterification, where ester bonds are formed rather than broken.
Mechanisms of Esterification
Esterification involves the reaction of a carboxylic acid with an alcohol, catalyzed by an acid, to form an ester and water through a nucleophilic acyl substitution mechanism. The mechanism begins with protonation of the carbonyl oxygen, increasing electrophilicity, followed by nucleophilic attack by the alcohol's hydroxyl group, and subsequent proton transfers lead to the elimination of water and formation of the ester. Hydrolysis, in contrast, reverses this process by breaking the ester bond in the presence of water and acid or base, regenerating the carboxylic acid and alcohol.
Key Differences Between Hydrolysis and Esterification
Hydrolysis and esterification are chemical reactions involving esters but differ fundamentally in direction and purpose. Hydrolysis breaks down esters into acids and alcohols using water, catalyzed by acid or base, while esterification synthesizes esters from acids and alcohols with the removal of water, typically catalyzed by acid. The key difference is hydrolysis is a cleavage process releasing components, whereas esterification is a combination process forming esters.
Common Examples in Organic Chemistry
Hydrolysis commonly occurs in the breakdown of esters such as the saponification of fats into glycerol and soap using a strong base. Esterification typically involves the reaction between a carboxylic acid and an alcohol, exemplified by the synthesis of ethyl acetate from acetic acid and ethanol under acidic conditions. Both processes are fundamental in organic chemistry for manipulating ester functional groups in laboratory and industrial applications.
Industrial Applications and Importance
Hydrolysis and esterification are vital chemical reactions extensively utilized in industrial applications, particularly in the production of biodiesel, pharmaceuticals, and polymers. Hydrolysis is crucial for breaking down complex esters into acids and alcohols, enabling the recycling of raw materials and wastewater treatment. Esterification plays a key role in manufacturing synthetic flavors, fragrances, and plasticizers, highlighting its importance in creating value-added products in the chemical industry.
Factors Influencing Reaction Rates
Reaction rates in hydrolysis and esterification are primarily influenced by factors such as temperature, pH, catalyst presence, and reactant concentration. Acid or base catalysts significantly accelerate both processes by stabilizing intermediates and lowering activation energy, while higher temperatures increase molecular collisions, enhancing reaction speed. The concentration of water and ester or carboxylic acid substrates directly affects the equilibrium position, thereby impacting the observed reaction rates in these reversible reactions.
Environmental and Biological Relevance
Hydrolysis and esterification are crucial biochemical reactions impacting environmental and biological systems by regulating the formation and breakdown of esters, which are vital in metabolism and cellular processes. Hydrolysis, involving the cleavage of ester bonds by water, facilitates the degradation of pollutants and organic matter, enhancing biodegradability and nutrient recycling in ecosystems. Esterification, the formation of esters from acids and alcohols, plays a significant role in synthesizing bioactive molecules and bioplastics, contributing to sustainable industrial applications and reducing reliance on fossil fuels.
Summary: Choosing Between Hydrolysis and Esterification
Hydrolysis breaks down esters into acids and alcohols, making it ideal for decomposition or recycling processes, while esterification synthesizes esters from acids and alcohols, commonly used in perfume and pharmaceutical production. The choice depends on the desired chemical transformation: hydrolysis favors bond cleavage, and esterification promotes bond formation. Reaction conditions such as catalysts, temperature, and pH also play critical roles in selecting between these two processes.
Hydrolysis Infographic
 libterm.com
libterm.com