Aldoses are simple sugars characterized by an aldehyde group at the end of their carbon chain, playing a crucial role in metabolism and energy production. These monosaccharides include glucose, galactose, and ribose, which are essential for cellular functions and genetic material formation. Discover how understanding aldoses can impact Your knowledge of biochemistry by exploring the rest of this article.
Table of Comparison
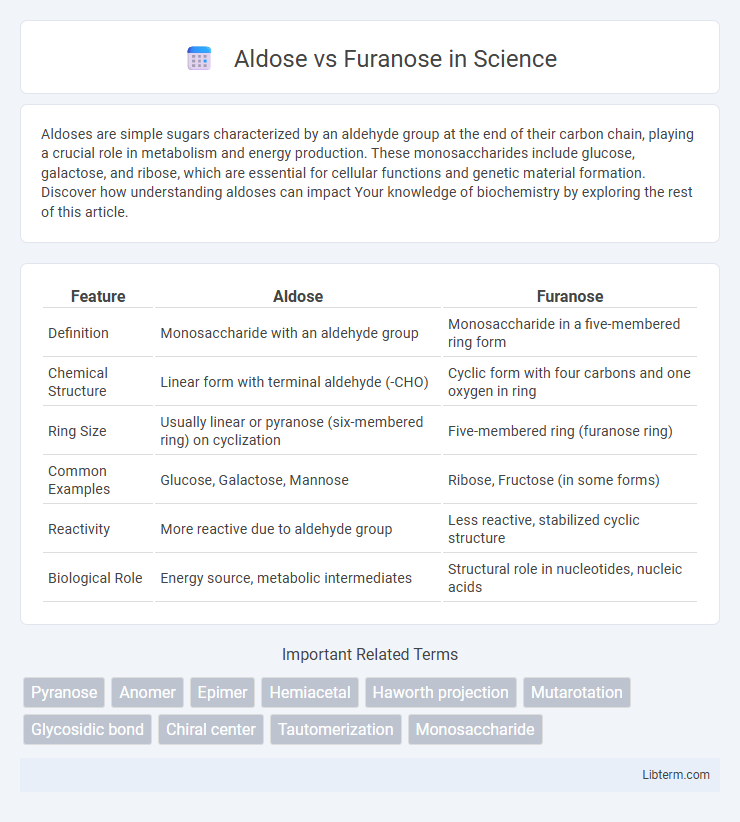
| Feature | Aldose | Furanose |
|---|---|---|
| Definition | Monosaccharide with an aldehyde group | Monosaccharide in a five-membered ring form |
| Chemical Structure | Linear form with terminal aldehyde (-CHO) | Cyclic form with four carbons and one oxygen in ring |
| Ring Size | Usually linear or pyranose (six-membered ring) on cyclization | Five-membered ring (furanose ring) |
| Common Examples | Glucose, Galactose, Mannose | Ribose, Fructose (in some forms) |
| Reactivity | More reactive due to aldehyde group | Less reactive, stabilized cyclic structure |
| Biological Role | Energy source, metabolic intermediates | Structural role in nucleotides, nucleic acids |
Introduction to Aldose and Furanose
Aldoses are monosaccharides containing an aldehyde group, playing key roles in energy metabolism and biosynthesis. Furanoses represent five-membered ring structures formed by the cyclization of sugars, often involving the reaction between an aldehyde or ketone group and a hydroxyl group. Understanding the structural differences between aldoses and furanoses aids in comprehending carbohydrate chemistry and their biological functions.
Chemical Structure Differences
Aldose sugars contain an aldehyde group (-CHO) at the terminal carbon, which defines their open-chain form and reactivity. Furanose forms arise when the sugar cyclizes into a five-membered ring comprising four carbons and one oxygen atom, significantly altering the spatial arrangement of hydroxyl groups. The primary structural difference lies in the presence of the aldehyde group in aldoses versus the cyclic ether linkage in the furanose ring structure, influencing their chemical behavior and interaction with other molecules.
Formation Mechanisms
Aldose sugars form via the oxidation of the aldehyde group at the C1 carbon, resulting in a linear structure that can cyclize through nucleophilic attack by the hydroxyl group on the C4 or C5 carbon, leading to ring closure. Furanose forms specifically when the hydroxyl on the C4 carbon attacks the aldehyde carbon, creating a five-membered ring structure characterized by four carbons and one oxygen atom. This intramolecular cyclization stabilizes the sugar molecule, influencing its chemical reactivity and biological function.
Functional Groups and Reactivity
Aldoses contain an aldehyde functional group (-CHO) at the terminal carbon, which contributes to their high reactivity, particularly in oxidation-reduction reactions and sugar identification tests. Furanoses are cyclic forms of sugars featuring a five-membered ring with an ether linkage, where the aldehyde group forms a hemiacetal structure, reducing free aldehyde reactivity but increasing susceptibility to ring-opening reactions. The presence of the aldehyde in aldoses makes them more reactive towards nucleophiles, while the furanose ring stability affects intramolecular reactions and enzymatic recognition in metabolic pathways.
Common Examples in Nature
Glucose commonly exists in nature as both an aldose in its linear form and as a furanose when cyclized into a five-membered ring. Ribose, a crucial sugar in RNA, predominantly adopts a furanose structure due to its five-carbon backbone forming a stable five-membered ring. These examples highlight the structural diversity of carbohydrates that impact their biological functions and molecular recognition.
Biological Roles and Importance
Aldoses function primarily as essential energy sources and metabolic intermediates in cellular processes such as glycolysis and the pentose phosphate pathway. Furanoses, characterized by their five-membered ring structure, play critical roles in nucleic acid composition, forming the sugar backbone of RNA and DNA through ribose and deoxyribose. Both aldoses and furanoses enable key biological mechanisms including enzyme recognition, genetic information storage, and cellular signaling pathways.
Analytical Identification Methods
Aldose and furanose structures can be differentiated using nuclear magnetic resonance (NMR) spectroscopy, where chemical shift patterns reveal the presence of aldehyde groups in aldoses versus the cyclic ether ring in furanoses. High-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS) enables separation and mass-based identification, distinguishing aldoses by their open-chain form and furanoses by their five-membered ring. Infrared (IR) spectroscopy detects characteristic carbonyl absorption in aldoses around 1730 cm^-1, which is absent or shifted in the furanose ring, facilitating their analytical identification.
Aldose vs Furanose in Metabolism
Aldoses and furanoses play distinct roles in carbohydrate metabolism, with aldoses acting primarily as linear glucose forms involved in glycolysis and the pentose phosphate pathway. Furanoses, cyclic five-membered ring structures, are critical in nucleotide synthesis and energy transfer processes, exemplified by ribose in RNA and ATP. The interconversion between aldose and furanose forms enables versatile metabolic functions essential for cellular energy balance and genetic material formation.
Industrial and Medical Applications
Aldose sugars, such as glucose, are widely utilized in industrial fermentation processes for bioethanol production and as precursors in pharmaceuticals due to their linear form facilitating enzymatic reactions. Furanose forms, present in ribose and fructose, play a critical role in nucleic acid structures, making them essential in medical applications like DNA/RNA synthesis and antiviral drug development. The cyclic nature of furanoses contributes to their stability and interaction with biomolecules, enhancing their use in targeted drug delivery and diagnostic assays.
Comparative Summary and Key Insights
Aldose sugars contain an aldehyde group at the first carbon, making them linear in structure, while furanose sugars form a five-membered ring with four carbons and one oxygen atom, leading to cyclic structures. Aldoses such as glucose frequently exist in equilibrium between their open-chain and cyclic forms, with the furanose ring being less common compared to the pyranose form; however, furanose rings are crucial in nucleotides and nucleic acids, like ribose in RNA. The key insight is that aldose configuration determines reactivity and biological function, whereas furanose ring formation influences stability, sugar conformation, and interaction within biomolecules.
Aldose Infographic
 libterm.com
libterm.com