Lipolysis is the metabolic process where your body breaks down stored fat into free fatty acids and glycerol to be used as energy. This critical function plays a key role in weight management and overall metabolic health by regulating fat reserves. Explore the article to understand how lipolysis works and the factors that influence its efficiency.
Table of Comparison
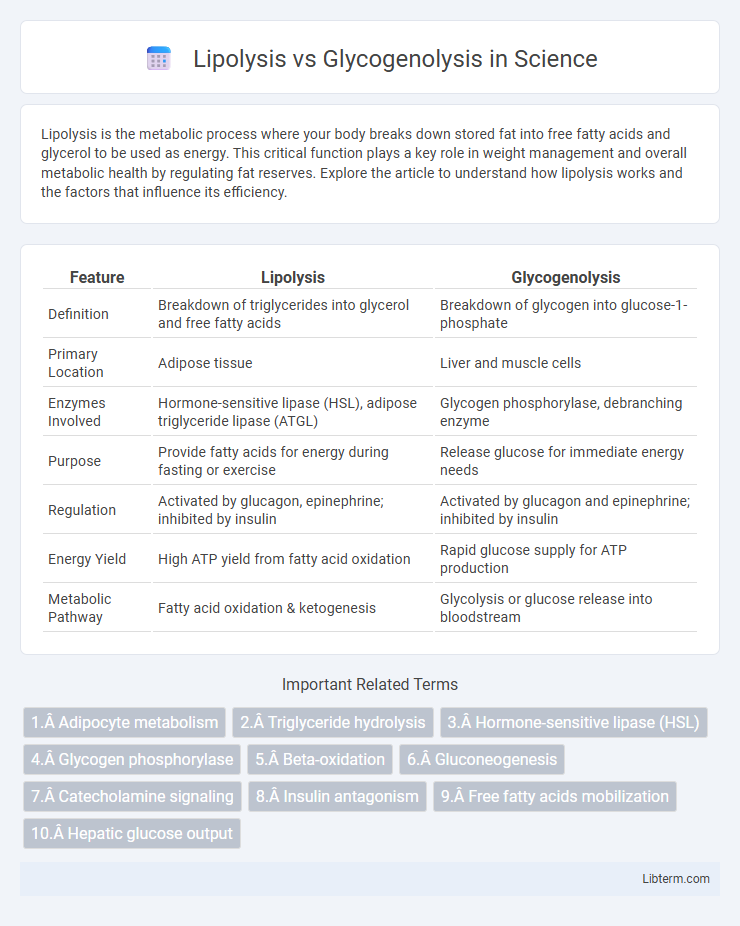
| Feature | Lipolysis | Glycogenolysis |
|---|---|---|
| Definition | Breakdown of triglycerides into glycerol and free fatty acids | Breakdown of glycogen into glucose-1-phosphate |
| Primary Location | Adipose tissue | Liver and muscle cells |
| Enzymes Involved | Hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL) | Glycogen phosphorylase, debranching enzyme |
| Purpose | Provide fatty acids for energy during fasting or exercise | Release glucose for immediate energy needs |
| Regulation | Activated by glucagon, epinephrine; inhibited by insulin | Activated by glucagon and epinephrine; inhibited by insulin |
| Energy Yield | High ATP yield from fatty acid oxidation | Rapid glucose supply for ATP production |
| Metabolic Pathway | Fatty acid oxidation & ketogenesis | Glycolysis or glucose release into bloodstream |
Introduction to Lipolysis and Glycogenolysis
Lipolysis is the metabolic process that breaks down triglycerides into glycerol and free fatty acids, providing energy during fasting or exercise, primarily in adipose tissue. Glycogenolysis involves the enzymatic degradation of glycogen into glucose-1-phosphate, supplying glucose to maintain blood sugar levels, especially in liver and muscle cells. Both processes are crucial for energy homeostasis, with lipolysis mobilizing fat stores and glycogenolysis rapidly releasing glucose from stored glycogen.
Definitions and Basic Concepts
Lipolysis is the metabolic process by which triglycerides are broken down into glycerol and free fatty acids, serving as a crucial energy source during fasting or prolonged exercise. Glycogenolysis involves the breakdown of glycogen stores into glucose-1-phosphate and then glucose-6-phosphate, providing rapid glucose availability for cellular energy demands. Both processes are essential for maintaining energy homeostasis, with lipolysis predominating during long-term energy deficits and glycogenolysis supplying immediate glucose during short-term energy needs.
Key Enzymes Involved in Lipolysis
Hormone-sensitive lipase (HSL) is the primary enzyme catalyzing the hydrolysis of stored triglycerides into free fatty acids and glycerol during lipolysis. Adipose triglyceride lipase (ATGL) initiates the breakdown of triglycerides by releasing the first fatty acid, while monoglyceride lipase (MGL) completes the process by hydrolyzing monoglycerides into glycerol. These enzymes coordinate to regulate energy mobilization from fat reserves, distinguishing lipolysis from glycogenolysis, which primarily depends on glycogen phosphorylase to break down glycogen into glucose-1-phosphate.
Key Enzymes Involved in Glycogenolysis
Glycogenolysis primarily involves key enzymes such as glycogen phosphorylase, which catalyzes the breakdown of glycogen into glucose-1-phosphate, and debranching enzyme that remodels glycogen to enable continued degradation. Phosphoglucomutase then converts glucose-1-phosphate into glucose-6-phosphate for entry into glycolysis or glucose release. In contrast, lipolysis relies on enzymes like hormone-sensitive lipase to hydrolyze triglycerides into free fatty acids and glycerol.
Biological Functions and Significance
Lipolysis is the metabolic process that breaks down triglycerides into free fatty acids and glycerol, providing essential energy during fasting or prolonged exercise. Glycogenolysis involves the enzymatic breakdown of glycogen into glucose-1-phosphate, supplying rapid glucose release to maintain blood sugar levels during short-term energy demands. Both pathways play critical roles in energy homeostasis, with lipolysis supporting long-term energy needs and glycogenolysis managing immediate glucose availability.
Regulation and Hormonal Control
Lipolysis is primarily regulated by hormones such as epinephrine, norepinephrine, and glucagon, which activate hormone-sensitive lipase to break down triglycerides into free fatty acids and glycerol for energy. Glycogenolysis is controlled mainly by glucagon and epinephrine, which stimulate glycogen phosphorylase to cleave glycogen into glucose-1-phosphate, providing rapid glucose release during fasting or stress. Insulin inhibits both lipolysis and glycogenolysis by promoting storage pathways and suppressing catabolic enzyme activity, maintaining metabolic balance.
Energy Yield: Lipolysis vs Glycogenolysis
Lipolysis breaks down triglycerides into free fatty acids and glycerol, producing significantly more ATP compared to glycogenolysis, which rapidly mobilizes glucose from glycogen stores. Each molecule of fatty acid undergoing beta-oxidation yields a large amount of ATP, approximately 106 ATP per palmitate, whereas glycogenolysis yields about 3 to 4 ATP per glucose unit released. This makes lipolysis a more efficient long-term energy source, while glycogenolysis provides quick but short-lived energy during high-intensity activities.
Conditions Triggering Each Pathway
Lipolysis is primarily triggered by low insulin levels, increased glucagon, and adrenaline during fasting, prolonged exercise, or stress, promoting the breakdown of triglycerides into free fatty acids and glycerol for energy. Glycogenolysis occurs in response to hormonal signals such as glucagon and epinephrine during short-term energy demands like sudden physical activity or hypoglycemia, breaking down glycogen stores into glucose. Both pathways are regulated to maintain energy homeostasis but activate under distinct metabolic conditions to supply appropriate fuel sources.
Clinical Relevance and Disorders
Lipolysis and glycogenolysis play critical roles in energy metabolism with distinct clinical implications; impaired lipolysis is linked to obesity, insulin resistance, and type 2 diabetes due to defective breakdown of triglycerides into free fatty acids. Glycogenolysis dysfunction, as seen in glycogen storage diseases (e.g., Von Gierke's disease), causes abnormal glycogen accumulation leading to hypoglycemia, hepatomegaly, and muscle weakness. Understanding these pathways aids in diagnosing metabolic disorders and developing targeted therapies for managing conditions like diabetes and glycogen storage diseases.
Summary: Comparing Lipolysis and Glycogenolysis
Lipolysis and glycogenolysis are critical metabolic processes that mobilize energy stores, with lipolysis breaking down triglycerides into free fatty acids and glycerol, while glycogenolysis involves the breakdown of glycogen into glucose-1-phosphate. Lipolysis primarily supports long-term energy demands by providing fatty acids for beta-oxidation, whereas glycogenolysis supplies rapid glucose availability for immediate energy requirements, especially in muscle and liver tissues. Enzyme activation differs as hormone-sensitive lipase drives lipolysis in adipose tissue, and glycogen phosphorylase catalyzes glycogenolysis in response to glucagon and epinephrine signaling.
Lipolysis Infographic
 libterm.com
libterm.com