An inhibitor is a substance that decreases the rate of a chemical reaction by interfering with the process, often by binding to an enzyme and reducing its activity. Understanding how inhibitors function can aid in designing drugs and controlling biochemical pathways effectively. Explore the rest of the article to learn how inhibitors impact various industries and your daily life.
Table of Comparison
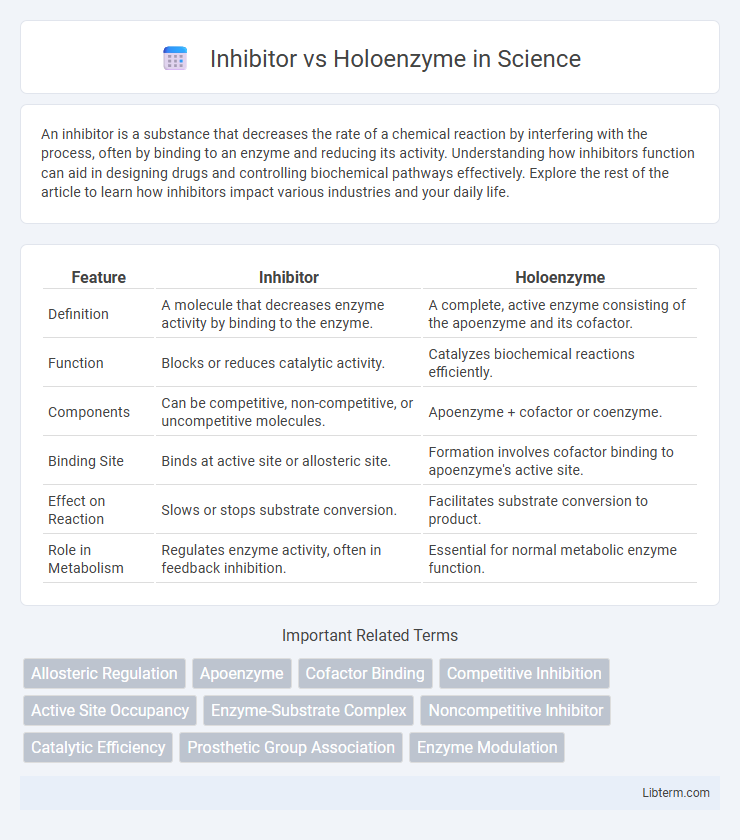
| Feature | Inhibitor | Holoenzyme |
|---|---|---|
| Definition | A molecule that decreases enzyme activity by binding to the enzyme. | A complete, active enzyme consisting of the apoenzyme and its cofactor. |
| Function | Blocks or reduces catalytic activity. | Catalyzes biochemical reactions efficiently. |
| Components | Can be competitive, non-competitive, or uncompetitive molecules. | Apoenzyme + cofactor or coenzyme. |
| Binding Site | Binds at active site or allosteric site. | Formation involves cofactor binding to apoenzyme's active site. |
| Effect on Reaction | Slows or stops substrate conversion. | Facilitates substrate conversion to product. |
| Role in Metabolism | Regulates enzyme activity, often in feedback inhibition. | Essential for normal metabolic enzyme function. |
Introduction to Enzyme Regulation
Enzyme regulation involves controlling enzyme activity through molecules like inhibitors and holoenzymes, which modulate catalytic efficiency and metabolic pathways. Inhibitors are molecules that decrease enzyme activity by binding to active or allosteric sites, often used to control reaction rates or signal pathways. Holoenzymes represent the complete, active form of an enzyme bound to its necessary cofactors or coenzymes, essential for proper catalytic function and regulation.
Defining Inhibitors in Enzymatic Processes
Inhibitors in enzymatic processes are molecules that decrease or prevent enzyme activity by binding to the enzyme, often altering its function or blocking substrate access. Unlike holoenzymes, which are fully functional enzymes composed of an apoenzyme and necessary cofactors or coenzymes, inhibitors interfere with the enzyme's catalytic capabilities without contributing to catalysis. Understanding enzyme inhibitors is crucial for drug design and metabolic regulation, as they provide insight into controlling enzyme activity through competitive, non-competitive, or allosteric mechanisms.
What is a Holoenzyme?
A holoenzyme is a fully functional enzyme complex consisting of a core enzyme and its associated cofactors or regulatory subunits. This complete structure enables the holoenzyme to perform precise catalytic activities necessary for biochemical reactions. Unlike inhibitors that block enzyme function, a holoenzyme facilitates substrate binding and catalysis, ensuring efficient metabolic processes.
Structural Differences: Inhibitor vs Holoenzyme
Inhibitors typically bind to specific sites on enzymes, altering their structural conformation and preventing substrate interaction or catalytic activity, whereas holoenzymes are fully assembled enzyme complexes consisting of a core enzyme bound to regulatory subunits or cofactors necessary for their functional conformation. The holoenzyme's structure facilitates substrate binding and catalysis, while inhibitors induce conformational changes that disrupt these processes. Structural differences include the presence of inhibitor molecules occupying active or allosteric sites in inhibited forms, contrasting with the intact, active architecture of holoenzyme assemblies.
Mechanisms of Action: How Inhibitors and Holoenzymes Operate
Inhibitors function by binding to enzymes to reduce their activity either competitively at the active site or non-competitively at allosteric sites, thereby altering the enzyme's conformation and preventing substrate interaction. Holoenzymes operate as active enzyme complexes formed by the association of a core enzyme and a required cofactor or coenzyme, facilitating precise catalytic activity and substrate specificity. The mechanisms of action involve inhibitors disrupting enzymatic catalysis, while holoenzymes ensure full enzymatic functionality through structural assembly and cofactor integration.
Functional Roles in Cellular Metabolism
Inhibitors regulate enzyme activity by binding to enzymes and decreasing their catalytic efficiency, thus modulating metabolic pathways and maintaining cellular homeostasis. Holoenzymes, composed of an apoenzyme and its cofactor, are fully functional complexes essential for catalyzing specific biochemical reactions in metabolism. The dynamic interplay between inhibitors and holoenzymes controls metabolic flux, ensuring precise regulation of energy production and biosynthesis within the cell.
Types of Enzyme Inhibition
Enzyme inhibition is classified into reversible and irreversible types, with reversible inhibition further divided into competitive, non-competitive, uncompetitive, and mixed inhibition based on the inhibitor's interaction with the enzyme or enzyme-substrate complex. Inhibitors bind to the enzyme, altering its activity by blocking the active site or binding allosterically, whereas holoenzymes represent the active form of an enzyme combined with its necessary cofactors or coenzymes. Understanding these inhibition types is crucial for drug design and regulating enzyme function in various biochemical pathways.
Holoenzyme Assembly and Regulation
Holoenzyme assembly involves the precise binding of a core enzyme with specific regulatory subunits, enabling complete catalytic activity essential for processes like transcription and metabolic regulation. Inhibitors target either the core enzyme or regulatory components, modulating holoenzyme function by preventing proper assembly or altering conformation, thus controlling enzymatic activity. Understanding the dynamics of holoenzyme formation and its inhibition provides critical insights into cellular regulation and therapeutic strategies against disease-related enzyme dysfunction.
Therapeutic Applications: Targeting Inhibitors and Holoenzymes
Targeting enzyme inhibitors and holoenzymes offers promising therapeutic applications by selectively modulating enzyme activity involved in disease pathways. Inhibitors can bind to active sites or allosteric regions of holoenzymes, effectively reducing pathological enzyme activity in conditions such as cancer, infectious diseases, and neurodegeneration. Precision design of small molecule inhibitors or monoclonal antibodies enhances specificity towards holoenzyme complexes, improving treatment efficacy while minimizing off-target effects.
Summary: Comparing Inhibitors and Holoenzymes
Inhibitors are molecules that bind to enzymes to reduce or halt their catalytic activity, often by blocking substrate binding or altering the enzyme's conformation. Holoenzymes consist of the apoenzyme and necessary cofactors or coenzymes, enabling full enzymatic activity and substrate processing. Comparing inhibitors and holoenzymes highlights the contrast between blockage of function versus facilitation of enzymatic reactions through complex formation.
Inhibitor Infographic
 libterm.com
libterm.com