Reduction is the process of decreasing size, quantity, or intensity to improve efficiency or solve problems. It plays a crucial role in sustainability, cost-cutting, and waste management across industries. Discover how embracing reduction strategies can transform your approach by reading the full article.
Table of Comparison
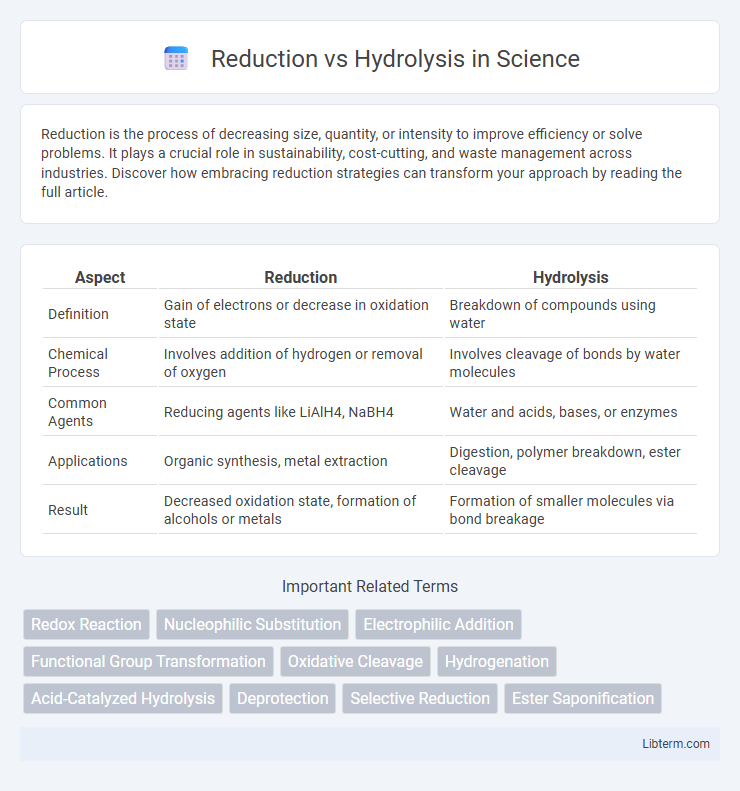
| Aspect | Reduction | Hydrolysis |
|---|---|---|
| Definition | Gain of electrons or decrease in oxidation state | Breakdown of compounds using water |
| Chemical Process | Involves addition of hydrogen or removal of oxygen | Involves cleavage of bonds by water molecules |
| Common Agents | Reducing agents like LiAlH4, NaBH4 | Water and acids, bases, or enzymes |
| Applications | Organic synthesis, metal extraction | Digestion, polymer breakdown, ester cleavage |
| Result | Decreased oxidation state, formation of alcohols or metals | Formation of smaller molecules via bond breakage |
Understanding Reduction and Hydrolysis
Reduction involves the gain of electrons or a decrease in oxidation state in a molecule, often transforming functional groups such as ketones into alcohols through the addition of hydrogen atoms. Hydrolysis is a chemical process that breaks bonds in molecules by the addition of water, commonly splitting esters, amides, and polysaccharides into smaller components. Both reactions play crucial roles in organic chemistry, with reduction altering oxidation levels and hydrolysis facilitating molecular cleavage via water incorporation.
Defining Reduction: Key Concepts
Reduction involves the gain of electrons or hydrogen atoms by a molecule, often resulting in a decrease in oxidation state. This key concept contrasts with hydrolysis, which entails the cleavage of chemical bonds through the addition of water. Understanding the electron transfer and molecular changes in reduction is fundamental to redox reactions and metabolic pathways.
What is Hydrolysis? An Overview
Hydrolysis is a chemical reaction involving the cleavage of bonds in a molecule through the addition of water, commonly breaking down complex molecules into simpler units. This process is essential in biological systems for digesting polymers like carbohydrates, proteins, and lipids into absorbable monomers. Compared to reduction, which involves the gain of electrons or hydrogen atoms, hydrolysis specifically utilizes water to split chemical bonds, playing a critical role in metabolism and industrial applications.
Chemical Mechanisms of Reduction
Reduction involves the gain of electrons or hydrogen atoms by a molecule, often facilitated by reducing agents such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4). The chemical mechanism typically proceeds through nucleophilic attack on an electrophilic carbon center, converting carbonyl groups (C=O) into alcohols (C-OH) via hydride transfer. In contrast, hydrolysis breaks chemical bonds through the addition of water, often cleaving esters, amides, or glycosidic linkages, without involving electron transfer processes characteristic of reduction.
Chemical Mechanisms of Hydrolysis
Hydrolysis involves the cleavage of chemical bonds through the addition of water molecules, typically targeting esters, amides, and glycosidic linkages in organic compounds. The chemical mechanism of hydrolysis often proceeds via nucleophilic attack by a hydroxide ion or water molecule on an electrophilic carbon, leading to bond cleavage and formation of hydroxyl and protonated species. In contrast to reduction, which involves electron gain and decreases oxidation states, hydrolysis changes molecular structure primarily through bond breaking without altering overall oxidation numbers.
Biological Importance of Reduction
Reduction reactions in biological systems play a critical role in cellular metabolism by enabling the gain of electrons essential for energy production and synthesis of biomolecules. Enzymes such as dehydrogenases facilitate reduction processes in pathways like glycolysis and the electron transport chain, driving ATP generation and maintaining cellular redox balance. These reduction reactions are vital for detoxifying reactive oxygen species and supporting biosynthetic reactions, underscoring their importance in sustaining life.
Biological Roles of Hydrolysis
Hydrolysis plays a crucial biological role by breaking down complex molecules like proteins, carbohydrates, and lipids into smaller, absorbable units through enzymatic action such as proteases, amylases, and lipases. This process is essential for nutrient absorption, cellular metabolism, and energy production within organisms. Unlike reduction reactions that alter oxidation states in biochemical pathways, hydrolysis primarily facilitates digestion, molecular recycling, and signal transduction in living systems.
Comparative Analysis: Reduction vs Hydrolysis
Reduction involves the gain of electrons or hydrogen atoms to decrease the oxidation state of a molecule, often converting carbonyl groups to alcohols. Hydrolysis breaks chemical bonds through the addition of water, commonly splitting esters or amides into acids and alcohols or amines. Comparative analysis reveals that reduction alters molecular oxidation levels and functional groups, while hydrolysis targets bond cleavage via water without changing oxidation states.
Applications in Industry and Research
Reduction processes are extensively applied in the chemical industry for the synthesis of pharmaceuticals, agrochemicals, and polymers, facilitating the conversion of functional groups to more reactive or stable forms. Hydrolysis reactions play a critical role in biotechnological research and industrial processes such as enzyme catalysis, biomass conversion, and the production of biofuels by breaking down complex molecules into simpler, functional units. Both reduction and hydrolysis are fundamental for developing sustainable chemical processes, enabling catalyst design and environmental remediation technologies.
Choosing Between Reduction and Hydrolysis Methods
Choosing between reduction and hydrolysis methods depends on the specific chemical transformation required: reduction involves the gain of electrons or hydrogen to convert functional groups like ketones or aldehydes into alcohols, while hydrolysis breaks bonds through reaction with water, typically cleaving esters, amides, or glycosidic linkages. For selective conversion of carbonyl compounds to alcohols, catalytic hydrogenation or metal hydride reagents such as sodium borohydride are preferred reduction techniques. Hydrolysis is optimal for achieving bond cleavage in polymers, peptides, or carbohydrates, making acid-, base-, or enzyme-catalyzed hydrolysis methods ideal for disassembly or functional group liberation.
Reduction Infographic
 libterm.com
libterm.com