Element plays a crucial role in chemistry, representing a pure substance consisting of one type of atom defined by its atomic number. Understanding how elements interact forms the foundation of studying compounds and chemical reactions essential in both science and industry. Explore further to discover how elements influence your everyday life and the world around you.
Table of Comparison
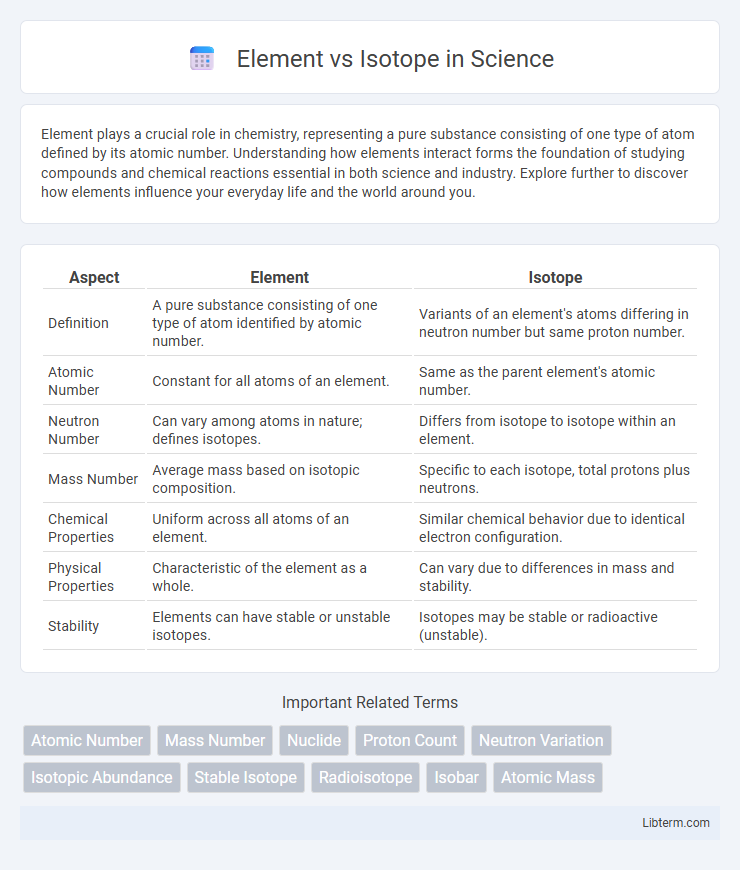
| Aspect | Element | Isotope |
|---|---|---|
| Definition | A pure substance consisting of one type of atom identified by atomic number. | Variants of an element's atoms differing in neutron number but same proton number. |
| Atomic Number | Constant for all atoms of an element. | Same as the parent element's atomic number. |
| Neutron Number | Can vary among atoms in nature; defines isotopes. | Differs from isotope to isotope within an element. |
| Mass Number | Average mass based on isotopic composition. | Specific to each isotope, total protons plus neutrons. |
| Chemical Properties | Uniform across all atoms of an element. | Similar chemical behavior due to identical electron configuration. |
| Physical Properties | Characteristic of the element as a whole. | Can vary due to differences in mass and stability. |
| Stability | Elements can have stable or unstable isotopes. | Isotopes may be stable or radioactive (unstable). |
Understanding Elements: The Basics
Elements consist of atoms with the same number of protons, defining their chemical properties and atomic number. Isotopes are variants of a single element that differ in neutron count, resulting in different atomic masses but identical chemical behavior. Understanding the distinction between elements and isotopes is crucial for applications in chemistry, nuclear physics, and radiometric dating.
What Are Isotopes?
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons in their atomic nuclei. This variation causes isotopes of an element to have identical atomic numbers but different mass numbers. Isotopes can be stable or radioactive, influencing their applications in fields such as medicine, archaeology, and nuclear energy.
Key Differences Between Elements and Isotopes
Elements are pure substances defined by their atomic number, representing the number of protons in the nucleus, while isotopes are variants of the same element with differing neutron counts but identical proton numbers. Elements exhibit unique chemical properties, whereas isotopes share chemical behavior but differ in nuclear properties such as stability and mass. Key differences include atomic mass variation in isotopes and consistent elemental identity determined solely by proton count.
Atomic Structure: Elements vs. Isotopes
Elements are defined by the number of protons in their atomic nucleus, which determines their unique atomic number and chemical properties. Isotopes of an element share the same number of protons but differ in neutron count, resulting in variations in atomic mass while maintaining identical chemical behavior. The difference in neutron number influences nuclear stability and can affect radioactive properties, but isotopes retain the element's fundamental identity.
Chemical Properties: Elemental vs. Isotopic Behavior
Elements exhibit consistent chemical properties due to their identical number of protons and electron configurations, which dictate reactivity and bonding patterns. Isotopes of the same element share these chemical properties because they possess the same number of protons and electrons, but differ in neutron count, causing variations primarily in nuclear stability rather than chemical behavior. While elemental identity determines chemical interactions, isotopic differences influence physical properties like atomic mass and nuclear decay rates without altering typical chemical reactivity.
Real-World Examples of Elements and Isotopes
Iron is an element commonly found in Earth's core and used in construction, while its isotope iron-60 is used in radiometric dating to study ancient geological events. Carbon, an essential element for life, has isotopes like carbon-12 and carbon-14, where carbon-14 is crucial for carbon dating archaeological artifacts. Uranium, a heavy element used in nuclear power, includes isotopes uranium-235 and uranium-238, with uranium-235 being key for nuclear reactors and weapons.
The Role of Isotopes in Science and Industry
Isotopes play a crucial role in science and industry by enabling precise tracing and analysis of chemical and biological processes, as their atoms of the same element differ in neutron number. Radioisotopes are extensively used in medical diagnostics and treatment, such as in PET scans and cancer radiotherapy, while stable isotopes assist in environmental studies and food authenticity verification. The unique properties of isotopes facilitate applications in archaeology through radiocarbon dating and in nuclear energy production via isotope enrichment.
Importance of Elements in Everyday Life
Elements such as carbon, oxygen, and hydrogen form the fundamental building blocks of all matter, making them indispensable to life and the environment. Isotopes, variants of elements differing in neutron number, play specialized roles in medical imaging, radiometric dating, and agricultural tracing, but the elements themselves are essential for biological processes, energy production, and material development. Understanding the unique properties of elements enables innovations in chemistry, medicine, and technology that drive daily comforts and scientific progress.
Isotopic Notation and Identification
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, affecting their atomic mass without changing chemical properties. Isotopic notation identifies isotopes by writing the element's symbol with the mass number (sum of protons and neutrons) as a superscript and the atomic number (number of protons) as a subscript, for example, 126C for Carbon-12. This notation aids in distinguishing isotopes for scientific analysis, such as in radiometric dating or nuclear medicine.
Element and Isotope Applications in Modern Technology
Elements serve as the fundamental building blocks in modern technology, with applications ranging from semiconductors in electronics to catalysts in chemical reactions. Isotopes, variants of elements with different neutron counts, enable advancements in medical imaging and cancer treatment through radioactive tracers and radiation therapy. Both elements and isotopes play critical roles in nuclear energy production, materials science, and environmental tracing technologies.
Element Infographic
 libterm.com
libterm.com