Acids are chemical substances characterized by their ability to donate protons or accept electron pairs, often resulting in a sour taste and a pH level below 7. Common acids like hydrochloric acid and citric acid play crucial roles in industrial processes and biological functions. Explore the rest of the article to deepen your understanding of acids and their practical applications in everyday life.
Table of Comparison
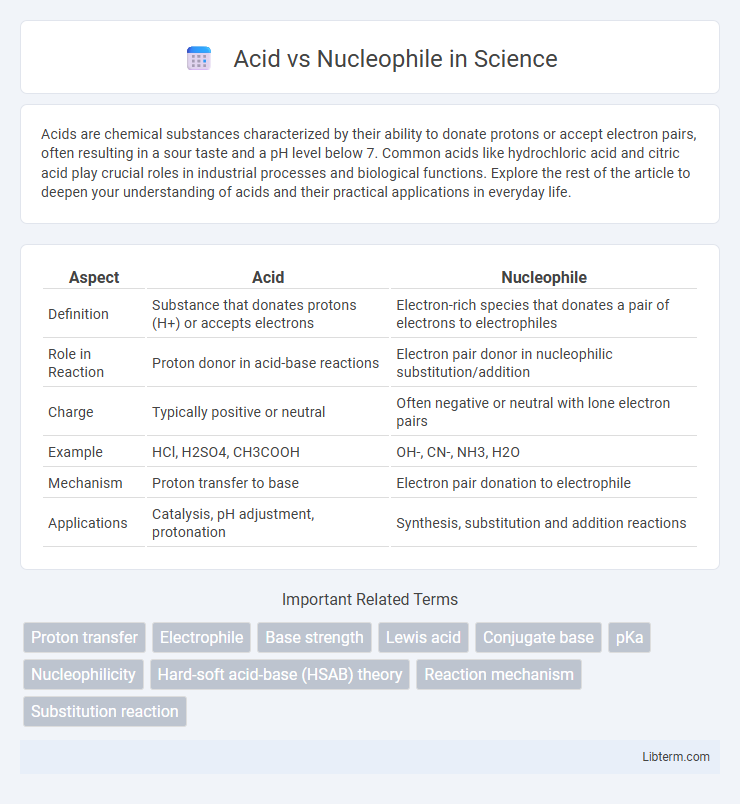
| Aspect | Acid | Nucleophile |
|---|---|---|
| Definition | Substance that donates protons (H+) or accepts electrons | Electron-rich species that donates a pair of electrons to electrophiles |
| Role in Reaction | Proton donor in acid-base reactions | Electron pair donor in nucleophilic substitution/addition |
| Charge | Typically positive or neutral | Often negative or neutral with lone electron pairs |
| Example | HCl, H2SO4, CH3COOH | OH-, CN-, NH3, H2O |
| Mechanism | Proton transfer to base | Electron pair donation to electrophile |
| Applications | Catalysis, pH adjustment, protonation | Synthesis, substitution and addition reactions |
Understanding Acids and Nucleophiles
Acids are proton donors that increase the concentration of hydrogen ions (H+) in a solution, while nucleophiles are electron-rich species that seek positively charged or electron-deficient centers to donate a pair of electrons. The strength of an acid is measured by its dissociation constant (Ka), whereas nucleophilicity depends on factors such as charge, electronegativity, solvent effects, and steric hindrance. Understanding the distinction between acid strength and nucleophilic reactivity is essential for predicting reaction mechanisms and outcomes in organic chemistry.
Defining Acid Strength and Reactivity
Acid strength is primarily defined by the molecule's tendency to donate protons, quantified by its acid dissociation constant (pKa), with lower pKa values indicating stronger acids. Nucleophile reactivity depends on electron density, charge, and steric hindrance, influencing its ability to donate electron pairs to electrophilic centers. The interplay between acid strength and nucleophile reactivity governs reaction mechanisms, where strong acids often facilitate protonation steps, while potent nucleophiles drive substitution and addition reactions.
Nucleophilicity: Meaning and Key Factors
Nucleophilicity refers to the ability of a nucleophile to donate a pair of electrons to an electrophile during a chemical reaction, making it a critical concept in organic chemistry. Key factors influencing nucleophilicity include electron density, solvent effects, steric hindrance, and the nucleophile's charge; for instance, negatively charged species typically exhibit higher nucleophilicity compared to their neutral counterparts. The solvent polarity also plays a significant role, with polar aprotic solvents enhancing nucleophilicity by not solvating the nucleophile as strongly as polar protic solvents.
Acid vs Nucleophile: Mechanistic Differences
Acids donate protons (H+) while nucleophiles donate electron pairs during chemical reactions, highlighting a fundamental mechanistic difference. Acid-base reactions involve proton transfer, whereas nucleophilic reactions involve the formation of new covalent bonds by nucleophiles attacking electrophilic centers. Understanding these mechanistic distinctions is crucial in predicting reaction pathways and designing synthetic strategies in organic chemistry.
Role in Chemical Reactions: Acids vs Nucleophiles
Acids act as proton donors, facilitating reactions by increasing the electrophilicity of substrates, while nucleophiles serve as electron pair donors, attacking electrophilic centers to form new bonds. In acid-catalyzed reactions, acids typically generate positively charged intermediates, enhancing reaction rates by stabilizing transition states. Conversely, nucleophiles directly participate in bond formation through electron pair donation, driving substitution and addition mechanisms in organic synthesis.
Examples of Common Acids and Nucleophiles
Common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH), which donate protons in chemical reactions. Typical nucleophiles comprise hydroxide ions (OH-), ammonia (NH3), and cyanide ions (CN-), characterized by their electron-rich nature seeking positively charged or electron-deficient centers. Understanding these examples aids in predicting reaction mechanisms and designing synthetic pathways in organic chemistry.
How Acids and Nucleophiles Interact in Reactions
Acids interact with nucleophiles by accepting electron pairs, forming covalent bonds during chemical reactions. Nucleophiles donate electron pairs to electrophilic acidic centers, facilitating bond formation and molecular transformations. This electron pair exchange is fundamental in mechanisms such as nucleophilic substitution and acid-base catalysis.
Factors Influencing Acidity and Nucleophilicity
Acidity is influenced by factors such as electronegativity, resonance stabilization, and the strength of the conjugate base, with more electronegative atoms and better resonance stabilization increasing acidity. Nucleophilicity depends on electron density, solvent effects, and steric hindrance, where higher electron density and less steric hindrance enhance nucleophilic strength. Solvent polarity plays a critical role, often increasing acidity by stabilizing ions while modulating nucleophilicity by affecting the availability of lone pairs for bonding.
Real-World Applications: From Synthesis to Industry
Acids and nucleophiles play pivotal roles in chemical synthesis and industrial processes, with acids often catalyzing reactions by protonating substrates, thereby increasing electrophilicity, while nucleophiles participate by donating electron pairs to form new chemical bonds. In pharmaceutical manufacturing, acid catalysis accelerates esterification and hydrolysis steps, whereas nucleophiles enable the formation of complex molecular architectures through substitution and addition reactions. Industrial applications such as polymer production and agrochemical synthesis exploit the distinct reactivities of acids and nucleophiles to optimize product yield, selectivity, and process efficiency.
Summary: Choosing Between Acid and Nucleophile
Choosing between an acid and a nucleophile depends primarily on the reaction context and substrate. Acids donate protons (H+) and are crucial in protonation steps, while nucleophiles donate electron pairs to form new bonds. Understanding the electrophilicity of the substrate and the strength of the nucleophile guides the selection for optimal reaction pathways.
Acid Infographic
 libterm.com
libterm.com