Diffusion is the natural movement of particles from an area of higher concentration to one of lower concentration, playing a crucial role in processes like gas exchange and nutrient absorption. Understanding diffusion helps you grasp essential biological and chemical phenomena that affect everyday life. Explore the rest of the article to learn how diffusion impacts various scientific fields and practical applications.
Table of Comparison
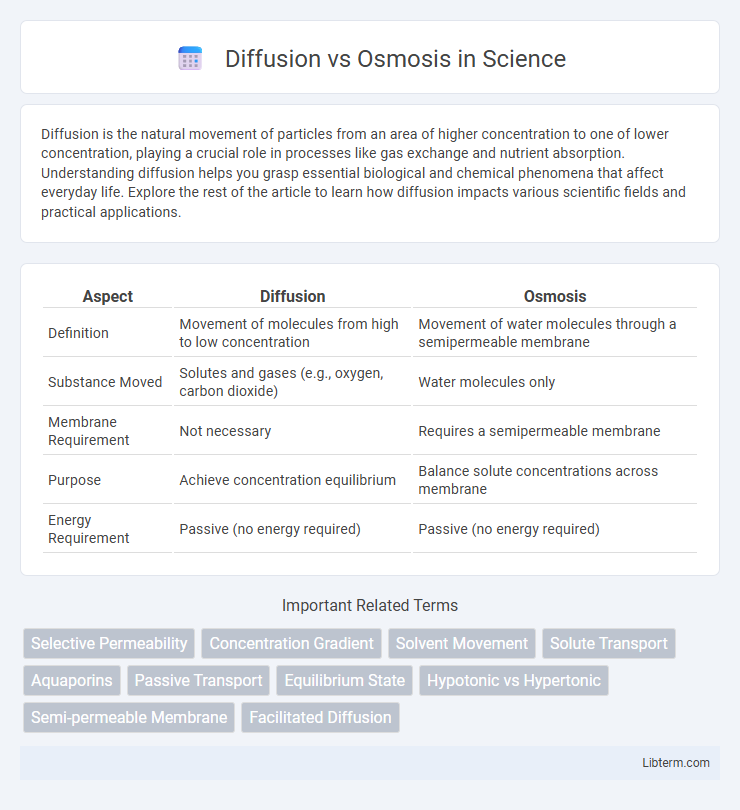
| Aspect | Diffusion | Osmosis |
|---|---|---|
| Definition | Movement of molecules from high to low concentration | Movement of water molecules through a semipermeable membrane |
| Substance Moved | Solutes and gases (e.g., oxygen, carbon dioxide) | Water molecules only |
| Membrane Requirement | Not necessary | Requires a semipermeable membrane |
| Purpose | Achieve concentration equilibrium | Balance solute concentrations across membrane |
| Energy Requirement | Passive (no energy required) | Passive (no energy required) |
Introduction to Diffusion and Osmosis
Diffusion is the passive movement of molecules from an area of higher concentration to an area of lower concentration, driven by the concentration gradient. Osmosis is a specific type of diffusion involving the movement of water molecules across a semipermeable membrane, moving from a region of low solute concentration to high solute concentration. Both processes are essential for maintaining cellular homeostasis and facilitate the transport of nutrients and waste in biological systems.
Defining Diffusion
Diffusion is the passive movement of molecules from an area of higher concentration to an area of lower concentration, driven by the concentration gradient. It occurs in gases, liquids, and solids, enabling the distribution of substances without requiring energy input. Diffusion is fundamental to processes such as gas exchange in lungs and nutrient absorption in cells.
Understanding Osmosis
Osmosis is the movement of water molecules through a semipermeable membrane from an area of low solute concentration to high solute concentration, aiming to equalize concentrations on both sides. Unlike diffusion, which involves the movement of solutes, osmosis specifically refers to the solvent's movement, primarily water, across membranes. This process is crucial for maintaining cell turgor pressure and regulating fluid balance in biological systems.
Fundamental Differences Between Diffusion and Osmosis
Diffusion involves the movement of particles from an area of higher concentration to lower concentration, occurring in gases, liquids, or solids without requiring a semipermeable membrane. Osmosis specifically refers to the diffusion of water molecules across a semipermeable membrane from a region of low solute concentration to high solute concentration, crucial for maintaining cellular homeostasis. The fundamental difference lies in diffusion's general particle movement versus osmosis' selective water movement through membranes.
Mechanisms of Molecular Movement
Diffusion is the passive movement of molecules from a region of higher concentration to lower concentration driven by the concentration gradient, occurring until equilibrium is reached. Osmosis specifically involves the movement of water molecules across a semipermeable membrane from an area of low solute concentration to high solute concentration to balance solute levels on both sides. Both processes rely on the kinetic energy of molecules but differ in their selective permeability and the types of molecules that move.
Role of Semi-Permeable Membranes
Semi-permeable membranes play a crucial role in osmosis by allowing only solvent molecules, typically water, to pass through while blocking solute particles, ensuring directional flow towards higher solute concentration. In diffusion, these membranes may not be necessary as molecules move freely from higher to lower concentration areas until equilibrium is reached. The selective permeability of these membranes differentiates osmosis from diffusion and is vital for maintaining cellular homeostasis.
Biological Significance of Diffusion
Diffusion plays a crucial role in cellular respiration by enabling oxygen to move from areas of high concentration in the lungs to lower concentration in blood cells, facilitating efficient gas exchange. It also supports nutrient absorption by allowing glucose and amino acids to passively enter cells where they are needed for metabolism and growth. Furthermore, diffusion helps in the removal of metabolic waste products like carbon dioxide, maintaining cellular homeostasis and overall physiological balance.
Biological Importance of Osmosis
Osmosis is crucial for maintaining cellular homeostasis by regulating water balance across semi-permeable membranes, enabling cells to absorb essential nutrients and remove waste efficiently. In biological systems, osmosis controls turgor pressure in plant cells, which supports structural integrity and growth. This process also facilitates kidney function by aiding in the reabsorption of water during urine formation, highlighting its vital role in organismal survival.
Real-World Examples in Cells and Organisms
Diffusion drives oxygen exchange in alveoli where oxygen molecules move from high concentration in the lungs into blood with lower oxygen levels. Osmosis regulates water balance in plant roots by moving water from soil with higher water potential into root cells having lower water potential, preventing dehydration. In animal cells, osmosis controls hydration by shifting water across semi-permeable membranes to maintain cellular turgor and homeostasis under varying external conditions.
Comparing Diffusion and Osmosis: Key Takeaways
Diffusion is the movement of molecules from an area of higher concentration to lower concentration, occurring in gases, liquids, and solids, while osmosis specifically involves the movement of water molecules through a semipermeable membrane. Both processes aim to achieve equilibrium but differ in their mediums and selective permeability. Understanding the distinctions between diffusion and osmosis is essential in fields such as biology, chemistry, and medical science for explaining cellular transport mechanisms.
Diffusion Infographic
 libterm.com
libterm.com