Autophagy is a critical cellular process that removes damaged components, maintaining cell health and preventing disease. This self-cleaning mechanism plays a vital role in aging, immunity, and metabolic regulation. Discover how autophagy can impact your well-being by exploring the full article.
Table of Comparison
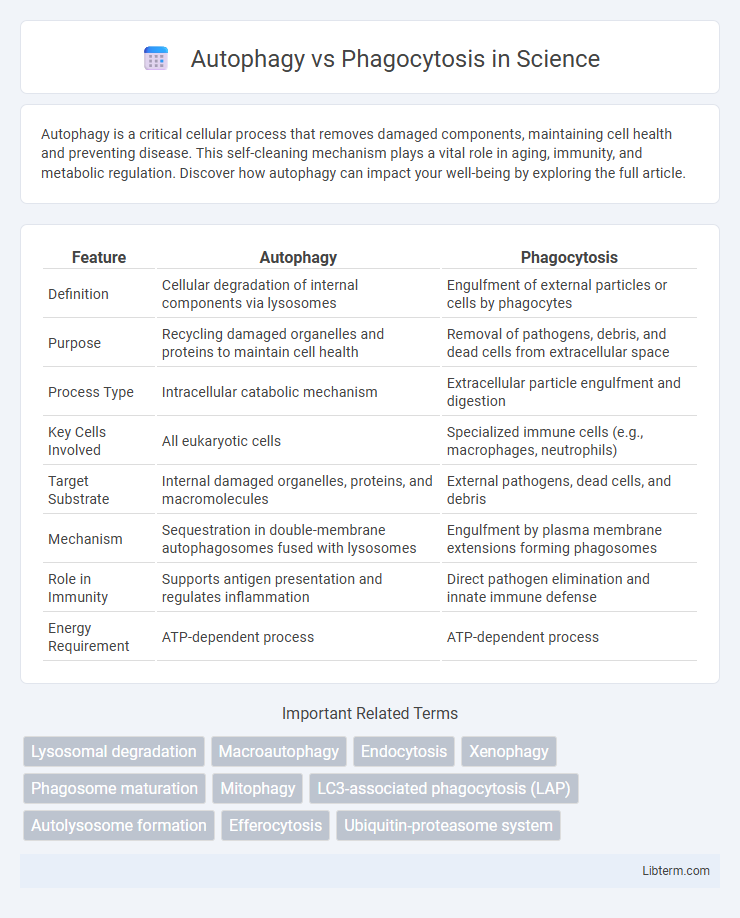
| Feature | Autophagy | Phagocytosis |
|---|---|---|
| Definition | Cellular degradation of internal components via lysosomes | Engulfment of external particles or cells by phagocytes |
| Purpose | Recycling damaged organelles and proteins to maintain cell health | Removal of pathogens, debris, and dead cells from extracellular space |
| Process Type | Intracellular catabolic mechanism | Extracellular particle engulfment and digestion |
| Key Cells Involved | All eukaryotic cells | Specialized immune cells (e.g., macrophages, neutrophils) |
| Target Substrate | Internal damaged organelles, proteins, and macromolecules | External pathogens, dead cells, and debris |
| Mechanism | Sequestration in double-membrane autophagosomes fused with lysosomes | Engulfment by plasma membrane extensions forming phagosomes |
| Role in Immunity | Supports antigen presentation and regulates inflammation | Direct pathogen elimination and innate immune defense |
| Energy Requirement | ATP-dependent process | ATP-dependent process |
Introduction to Cellular Degradation Pathways
Autophagy and phagocytosis are essential cellular degradation pathways that maintain homeostasis by removing damaged organelles, protein aggregates, and pathogens. Autophagy involves the formation of double-membrane autophagosomes that engulf intracellular components and fuse with lysosomes for degradation, playing a critical role in cellular quality control and energy balance. Phagocytosis, primarily performed by immune cells, entails the engulfment of extracellular particles or pathogens into phagosomes, which then fuse with lysosomes to degrade the contents and initiate immune responses.
Defining Autophagy: Mechanisms and Functions
Autophagy is a cellular degradation process that involves the lysosomal breakdown of damaged organelles and misfolded proteins to maintain cellular homeostasis and promote survival under stress conditions. It operates through the formation of double-membraned autophagosomes that engulf cytoplasmic material, which then fuse with lysosomes for enzymatic degradation. This mechanism is distinct from phagocytosis, which primarily targets extracellular particles for immune defense, while autophagy regulates intracellular quality control and metabolic adaptation.
Phagocytosis Overview: Process and Purpose
Phagocytosis is a cellular process where specialized cells, such as macrophages and neutrophils, engulf and digest extracellular particles including pathogens, dead cells, and debris to maintain tissue homeostasis and immune defense. This mechanism involves recognition of the target by cell surface receptors, engulfment into a phagosome, and subsequent fusion with lysosomes to degrade the contents. Phagocytosis plays a critical role in innate immunity by eliminating harmful microorganisms and facilitating antigen presentation to activate adaptive immune responses.
Key Differences Between Autophagy and Phagocytosis
Autophagy is a cellular process that degrades and recycles intracellular components, such as damaged organelles and proteins, through the formation of autophagosomes that fuse with lysosomes. Phagocytosis involves the engulfment of extracellular particles, pathogens, or debris by specialized cells like macrophages, forming phagosomes that subsequently fuse with lysosomes for degradation. Key differences include that autophagy targets intracellular components for homeostasis and stress responses, while phagocytosis primarily defends against external threats and clears extracellular material.
Cellular Triggers for Autophagy and Phagocytosis
Cellular triggers for autophagy primarily include nutrient deprivation, oxidative stress, and damaged organelles, which initiate the degradation and recycling of intracellular components to maintain cellular homeostasis. Phagocytosis is triggered by the recognition of extracellular particles such as pathogens, apoptotic cells, or debris through receptors like Fc receptors or pattern recognition receptors on the cell surface. These distinct stimuli activate specific signaling pathways to facilitate either intracellular self-digestion via autophagy or extracellular engulfment via phagocytosis.
Molecular Machinery: Autophagy vs Phagocytosis
Autophagy relies on the molecular machinery involving ATG proteins, such as ULK1 complex, Beclin-1, and LC3, which coordinate the formation of double-membrane autophagosomes to sequester cytoplasmic components for lysosomal degradation. In contrast, phagocytosis depends on surface receptors like Fc receptors and complement receptors to initiate actin-driven plasma membrane engulfment of extracellular particles into single-membrane phagosomes. The autophagy pathway emphasizes intracellular cargo recognition and recycling, while phagocytosis orchestrates extracellular pathogen or debris clearance through receptor-mediated signaling and cytoskeletal rearrangement.
Role in Immune Response and Homeostasis
Autophagy maintains cellular homeostasis by degrading damaged organelles and pathogens, enhancing antigen presentation and modulating inflammation to support immune responses. Phagocytosis directly eliminates extracellular pathogens and cellular debris, activating immune cells through pathogen recognition receptors to initiate inflammatory signaling. Both processes are crucial for immune defense and tissue homeostasis, with autophagy primarily regulating intracellular clearance and phagocytosis managing extracellular threats.
Pathological Implications of Dysfunctional Pathways
Dysfunctional autophagy is linked to neurodegenerative diseases such as Alzheimer's and Parkinson's, where impaired clearance of protein aggregates contributes to cellular toxicity. Defective phagocytosis disrupts immune responses, leading to chronic inflammation and autoimmune disorders like systemic lupus erythematosus. Both pathways' abnormalities are implicated in cancer progression by affecting cell survival, apoptosis, and immune evasion mechanisms.
Experimental Methods for Studying Autophagy and Phagocytosis
Experimental methods for studying autophagy include transmission electron microscopy (TEM) to visualize autophagosomes, Western blot analysis of LC3-II and p62 proteins as autophagy markers, and fluorescence microscopy using GFP-LC3 fusion proteins for monitoring autophagosome formation. Phagocytosis is commonly assessed through flow cytometry and fluorescence microscopy with labeled particles such as latex beads or bacteria to quantify engulfment and degradation. Advanced techniques like live-cell imaging and immunofluorescence staining further enable detailed examination of phagosome maturation and autophagic flux dynamics in various cellular contexts.
Therapeutic Potential: Targeting Autophagy and Phagocytosis
Targeting autophagy and phagocytosis offers promising therapeutic potential for treating various diseases, including cancer, neurodegenerative disorders, and infections. Modulating autophagy can enhance cellular clearance of damaged organelles and proteins, reducing pathological aggregates and improving cell survival. Phagocytosis-targeted therapies aim to boost immune response and clearance of pathogens or apoptotic cells, providing benefits in inflammatory diseases and immune dysfunction.
Autophagy Infographic
 libterm.com
libterm.com