Recombinant technology involves combining DNA from different sources to create new genetic combinations with valuable traits for medicine, agriculture, and industry. This technique enables the production of insulin, vaccines, and genetically modified crops, revolutionizing health and food security. Discover how recombinant methods can impact your life and drive innovation by reading the full article.
Table of Comparison
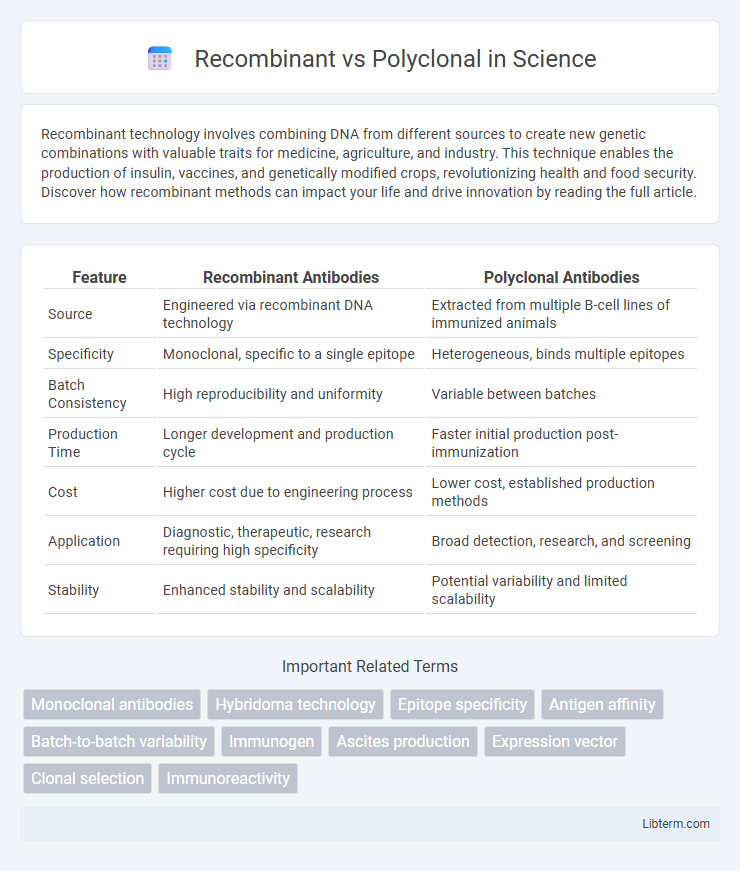
| Feature | Recombinant Antibodies | Polyclonal Antibodies |
|---|---|---|
| Source | Engineered via recombinant DNA technology | Extracted from multiple B-cell lines of immunized animals |
| Specificity | Monoclonal, specific to a single epitope | Heterogeneous, binds multiple epitopes |
| Batch Consistency | High reproducibility and uniformity | Variable between batches |
| Production Time | Longer development and production cycle | Faster initial production post-immunization |
| Cost | Higher cost due to engineering process | Lower cost, established production methods |
| Application | Diagnostic, therapeutic, research requiring high specificity | Broad detection, research, and screening |
| Stability | Enhanced stability and scalability | Potential variability and limited scalability |
Introduction to Recombinant and Polyclonal Antibodies
Recombinant antibodies are engineered by cloning specific antibody genes into expression systems, enabling high reproducibility and precise targeting of antigens. Polyclonal antibodies consist of a heterogeneous mix of antibodies produced by multiple B-cell clones in response to an antigen, offering broad epitope recognition and higher sensitivity. Recombinant antibodies provide consistency and scalability, whereas polyclonal antibodies excel in detecting complex epitopes due to their diverse binding profiles.
Defining Recombinant Antibodies
Recombinant antibodies are engineered proteins produced using DNA technology that allows precise control over their structure and specificity. Unlike polyclonal antibodies generated from multiple immune cell clones, recombinant antibodies are derived from a single gene sequence, ensuring consistency and reduced batch-to-batch variability. This specificity and reproducibility make recombinant antibodies ideal for diagnostic, therapeutic, and research applications requiring high precision.
Understanding Polyclonal Antibodies
Polyclonal antibodies consist of a mixture of immunoglobulin molecules secreted against a specific antigen, recognizing multiple epitopes, which enhances their binding versatility and sensitivity in diagnostic applications. They are produced by immunizing an animal and collecting serum containing diverse antibody populations, leading to batch variability but robust antigen recognition. Unlike recombinant antibodies, polyclonal antibodies may exhibit cross-reactivity due to their heterogeneous nature, making them ideal for applications requiring strong signal detection but less suitable for highly specific targeting.
Production Methods: Recombinant vs Polyclonal
Recombinant antibodies are produced using engineered DNA sequences cloned into expression systems such as bacteria, yeast, or mammalian cells, enabling precise control over antibody specificity and batch consistency. Polyclonal antibodies are generated by immunizing animals like rabbits or goats, leading to a heterogeneous mixture of antibodies targeting multiple epitopes on the antigen. Recombinant production offers scalable, reproducible yields with defined sequences, while polyclonal antibodies rely on biological responses, resulting in variable composition and limited scalability.
Specificity and Consistency Comparison
Recombinant antibodies exhibit high specificity due to their uniform genetic sequence, ensuring precise targeting of antigen epitopes with minimal cross-reactivity. Polyclonal antibodies, derived from multiple B-cell clones, offer broader epitope recognition but suffer from batch-to-batch variability, affecting consistency. Recombinant antibody production guarantees reproducible affinity and specificity across experiments, while polyclonal preparations may fluctuate in potency and target range.
Applications in Research and Diagnostics
Recombinant antibodies offer high specificity and consistency, making them ideal for precise research applications such as protein quantification and therapeutic target validation. Polyclonal antibodies provide broad epitope recognition, enhancing sensitivity in diagnostic assays like ELISA and immunohistochemistry for pathogen detection. Both types serve complementary roles, with recombinant antibodies preferred for reproducibility across experiments and polyclonal antibodies favored for robust detection of diverse antigen variants.
Advantages of Recombinant Antibodies
Recombinant antibodies offer remarkable advantages including batch-to-batch consistency, high specificity, and the ability to engineer antibody affinity and functionality precisely. These antibodies are produced using defined genetic sequences, eliminating variability found in traditional polyclonal antibodies derived from animal sera. Their renewable nature and reduced risk of contamination make recombinant antibodies ideal for reproducible research, diagnostics, and therapeutic applications.
Benefits and Limitations of Polyclonal Antibodies
Polyclonal antibodies offer the benefit of recognizing multiple epitopes on a single antigen, enhancing their sensitivity and effectiveness in detecting proteins with diverse structural forms. Their production is faster and more cost-effective compared to recombinant antibodies, making them suitable for research and diagnostic applications requiring broad specificity. However, polyclonal antibodies exhibit batch-to-batch variability and lower reproducibility, which can limit their consistency in long-term experiments and therapeutic use.
Factors Influencing Antibody Selection
Antibody selection is influenced by specificity, reproducibility, and batch-to-batch consistency, where recombinant antibodies offer precise epitope targeting through defined sequences, enhancing reliability in research and diagnostics. Polyclonal antibodies provide broad epitope recognition, beneficial for detecting proteins with multiple antigenic sites but suffer from variability due to heterogeneous antibody populations and limited batch uniformity. Factors such as application type, antigen complexity, and required validation standards determine whether the high specificity of recombinant antibodies or the diverse binding properties of polyclonal antibodies best suit experimental goals.
Future Trends in Antibody Development
Recombinant antibodies are rapidly gaining traction due to their consistency, scalability, and enhanced specificity compared to traditional polyclonal antibodies, which exhibit batch variability and limited reproducibility. Advances in phage display technology and next-generation sequencing fuel the development of recombinant antibodies with engineered affinities and tailored functionalities, addressing complex therapeutic and diagnostic needs. Future trends emphasize integrating artificial intelligence and machine learning to optimize antibody design and streamline high-throughput screening, driving precision medicine and novel biologics forward.
Recombinant Infographic
 libterm.com
libterm.com