Hydrolysis is a chemical process where water breaks down compounds into smaller molecules by cleaving bonds, playing a critical role in digestion and various industrial applications. This reaction is essential for converting complex substances like proteins and carbohydrates into absorbable units that Your body can utilize for energy and growth. Explore the full article to understand how hydrolysis impacts daily life and science.
Table of Comparison
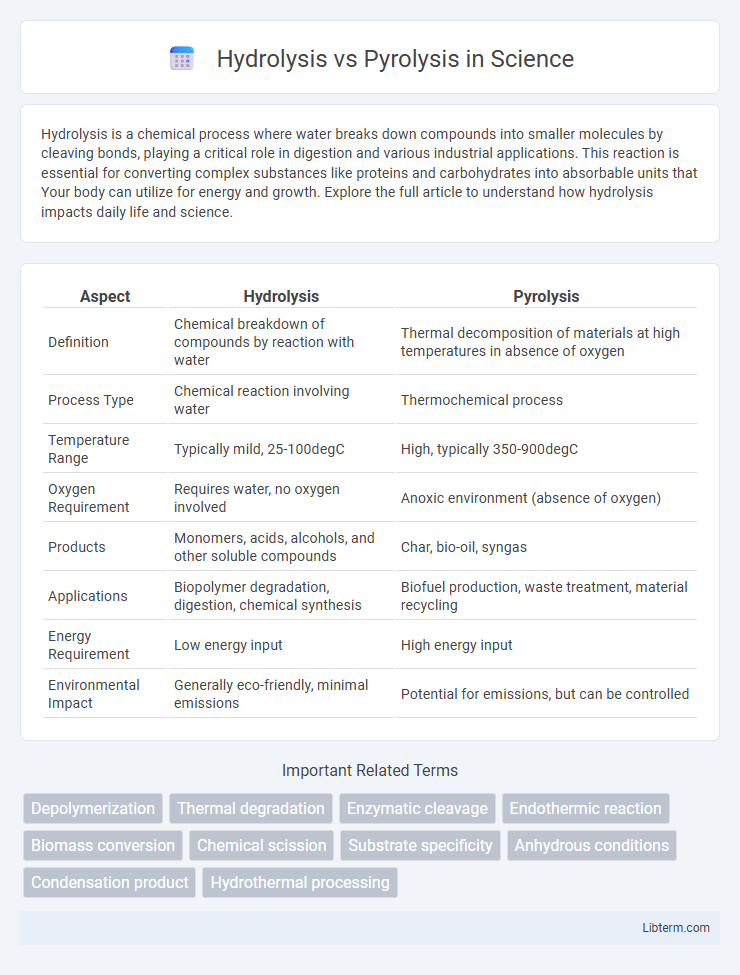
| Aspect | Hydrolysis | Pyrolysis |
|---|---|---|
| Definition | Chemical breakdown of compounds by reaction with water | Thermal decomposition of materials at high temperatures in absence of oxygen |
| Process Type | Chemical reaction involving water | Thermochemical process |
| Temperature Range | Typically mild, 25-100degC | High, typically 350-900degC |
| Oxygen Requirement | Requires water, no oxygen involved | Anoxic environment (absence of oxygen) |
| Products | Monomers, acids, alcohols, and other soluble compounds | Char, bio-oil, syngas |
| Applications | Biopolymer degradation, digestion, chemical synthesis | Biofuel production, waste treatment, material recycling |
| Energy Requirement | Low energy input | High energy input |
| Environmental Impact | Generally eco-friendly, minimal emissions | Potential for emissions, but can be controlled |
Introduction to Hydrolysis and Pyrolysis
Hydrolysis is a chemical process that involves the breakdown of compounds through the reaction with water, often catalyzed by acids, bases, or enzymes, making it essential in biochemistry and industrial applications. Pyrolysis, in contrast, involves the thermal decomposition of organic materials at high temperatures in the absence of oxygen, producing char, oil, and gas valuable for energy and material recovery. Both processes are critical in waste management and resource recovery, leveraging different mechanisms to convert organic substances into useful products.
Definition and Basic Principles
Hydrolysis is a chemical process that involves the cleavage of bonds in molecules through the addition of water, commonly used in breaking down complex carbohydrates, proteins, and fats. Pyrolysis is the thermal decomposition of organic materials at elevated temperatures in the absence of oxygen, leading to the production of bio-oil, syngas, and char. Both processes are fundamental in biomass conversion technologies, with hydrolysis relying on aqueous chemistry and enzymatic or acid catalysts, while pyrolysis depends on heat to induce molecular breakdown.
Key Differences Between Hydrolysis and Pyrolysis
Hydrolysis involves the chemical breakdown of compounds through reaction with water, commonly used to decompose complex molecules like carbohydrates and proteins into simpler units. Pyrolysis is the thermal decomposition of organic materials at elevated temperatures in the absence of oxygen, producing char, tar, and volatile gases. Key differences include hydrolysis requiring water and breaking chemical bonds via hydrolytic cleavage, while pyrolysis relies on heat without water and causes bond cleavage through thermal degradation.
Chemical Reactions Involved
Hydrolysis involves the chemical reaction where water molecules break down complex compounds into simpler substances by cleaving covalent bonds, typically in the presence of enzymes or acids. Pyrolysis, in contrast, is a thermal decomposition process occurring at high temperatures in an oxygen-free environment, breaking down organic materials into smaller molecules such as gases, oils, and char through bond cleavage. Both processes alter molecular structures fundamentally, with hydrolysis relying on water as a reactant and pyrolysis depending on heat-induced bond dissociation.
Types of Hydrolysis and Pyrolysis Processes
Hydrolysis involves breaking chemical bonds through the reaction with water, categorized into acid hydrolysis, base hydrolysis, and enzymatic hydrolysis, each differing by the catalyst or conditions used. Pyrolysis, the thermal decomposition of organic materials in the absence of oxygen, includes slow pyrolysis, fast pyrolysis, and flash pyrolysis, distinguished by heating rates and residence times that influence product yields such as biochar, bio-oil, and syngas. Understanding these types enables targeted applications in biofuel production, waste management, and chemical synthesis.
Applications in Industry
Hydrolysis is widely applied in the pharmaceutical and food industries for breaking down complex molecules like proteins and carbohydrates into simpler, usable components. Pyrolysis is extensively used in waste management and energy production to convert organic materials like biomass and plastics into biochar, syngas, and bio-oil. Both processes offer sustainable industrial solutions: hydrolysis for biochemical conversion and pyrolysis for thermal decomposition and resource recovery.
Environmental Impacts
Hydrolysis primarily produces biodegradable byproducts that reduce environmental toxicity, making it a more eco-friendly method for breaking down organic materials. Pyrolysis generates biochar and syngas, which can contribute to carbon sequestration and renewable energy production but also risks releasing harmful pollutants if not properly managed. Both processes impact soil and air quality differently, with hydrolysis favoring water-based ecosystems and pyrolysis influencing atmospheric emissions and soil enhancement.
Energy Requirements and Efficiency
Hydrolysis requires lower energy input as it typically occurs at moderate temperatures (30-100degC) and uses water to break chemical bonds, making it energy-efficient for biomass conversion. Pyrolysis demands significantly higher energy, operating at elevated temperatures (400-700degC) in the absence of oxygen, which increases energy consumption but produces a wider range of valuable products like bio-oil, syngas, and char. Energy efficiency in hydrolysis benefits from mild conditions and faster reaction rates, whereas pyrolysis efficiency depends on optimized heating rates and feedstock properties, resulting in trade-offs between energy input and product yield.
Advantages and Limitations
Hydrolysis offers the advantage of breaking down complex polymers into monomers using water and mild conditions, making it energy-efficient and environmentally friendly, but it often requires catalysts and longer reaction times. Pyrolysis, on the other hand, thermally decomposes organic material in the absence of oxygen at high temperatures, enabling rapid conversion into bio-oil, syngas, and char, yet it demands significant energy input and can produce hazardous byproducts. Both processes are crucial for biomass conversion, with hydrolysis favoring selectivity and sustainability, while pyrolysis excels in throughput and product diversity but with greater operational challenges.
Future Trends and Innovations
Hydrolysis advancements are increasingly centered on enzymatic processes and green chemistry to enhance efficiency in biomass conversion for sustainable biofuels and bioplastics production. Pyrolysis innovations emphasize improved thermal reactor designs and catalytic upgrading techniques to boost yield and selectivity of bio-oil and syngas from waste materials. Future trends include integrating artificial intelligence for process optimization and coupling both methods in hybrid systems to maximize energy recovery and reduce environmental impact.
Hydrolysis Infographic
 libterm.com
libterm.com