Freezing occurs when liquids turn solid due to temperature dropping below their freezing point, commonly observed with water solidifying into ice. This phase change affects numerous natural and industrial processes, from weather patterns to food preservation. Discover more about the mechanics of freezing and how it impacts your daily life in the detailed article ahead.
Table of Comparison
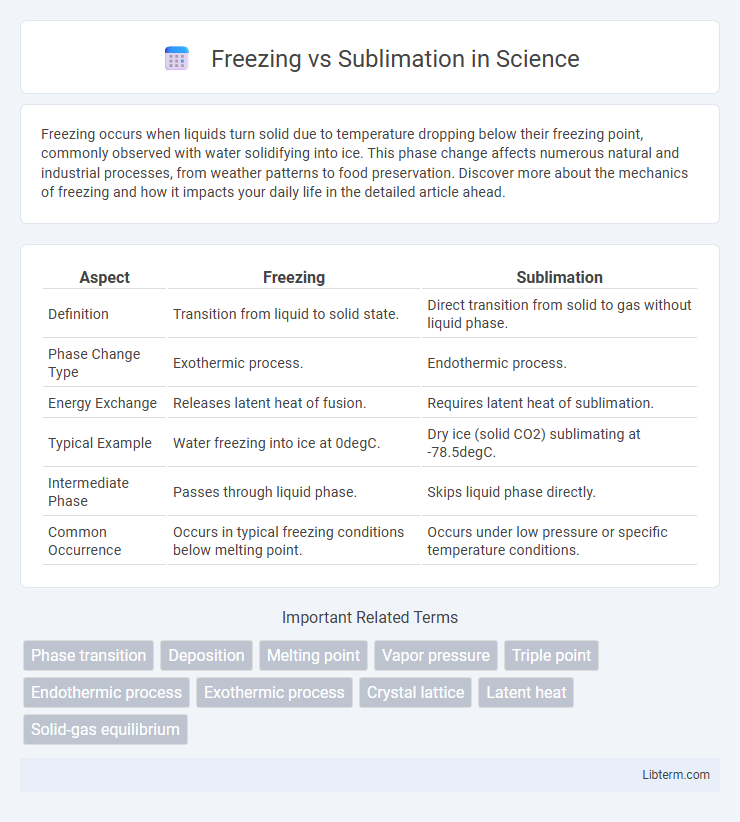
| Aspect | Freezing | Sublimation |
|---|---|---|
| Definition | Transition from liquid to solid state. | Direct transition from solid to gas without liquid phase. |
| Phase Change Type | Exothermic process. | Endothermic process. |
| Energy Exchange | Releases latent heat of fusion. | Requires latent heat of sublimation. |
| Typical Example | Water freezing into ice at 0degC. | Dry ice (solid CO2) sublimating at -78.5degC. |
| Intermediate Phase | Passes through liquid phase. | Skips liquid phase directly. |
| Common Occurrence | Occurs in typical freezing conditions below melting point. | Occurs under low pressure or specific temperature conditions. |
Understanding Phase Changes: Freezing and Sublimation
Freezing involves the phase transition where a liquid turns into a solid as its temperature decreases below its freezing point, resulting in a rigid molecular structure. Sublimation is the direct phase change from a solid to a gas without passing through the liquid state, typically occurring under low pressure and specific temperature conditions. Understanding the thermodynamic principles and energy exchanges during freezing and sublimation is essential for applications in materials science, meteorology, and industrial processes.
Defining Freezing: The Liquid to Solid Transition
Freezing is the phase transition where a liquid turns into a solid as its temperature drops below the substance's freezing point, causing molecules to slow down and arrange into a fixed, crystalline structure. This process releases latent heat of fusion, making it exothermic, and is commonly observed in water turning into ice at 0degC (32degF). Unlike sublimation, freezing involves an intermediate liquid phase rather than transitioning directly from gas to solid.
What is Sublimation? Solid to Gas Direct Conversion
Sublimation is the phase transition where a solid changes directly into a gas without passing through the liquid state, commonly observed in substances like dry ice (solid carbon dioxide) and iodine. This process occurs under specific temperature and pressure conditions, often below the substance's triple point on a phase diagram. In contrast, freezing involves the transition from liquid to solid, highlighting the unique direct solid-to-gas conversion characteristic of sublimation.
Key Differences Between Freezing and Sublimation
Freezing is the phase transition where a liquid changes into a solid by losing heat, typically occurring at the substance's freezing point. Sublimation bypasses the liquid phase as a solid transforms directly into a gas when exposed to specific temperature and pressure conditions, commonly seen with dry ice (solid CO2). The key difference lies in freezing requiring a liquid phase and heat removal, whereas sublimation involves a direct solid-to-gas change without passing through the liquid state.
The Science Behind Freezing: Temperature, Pressure, and Molecular Movement
Freezing occurs when a substance's temperature drops below its freezing point, causing molecules to slow down and arrange into a fixed, crystalline structure due to decreased molecular motion. Pressure influences the freezing point by altering molecular interactions; higher pressure can raise or lower the freezing temperature depending on the substance. Unlike sublimation, which bypasses the liquid state, freezing involves a clear phase transition from liquid to solid driven by thermal energy reduction and molecular bonding.
Sublimation Explained: Conditions and Examples
Sublimation occurs when a substance transitions directly from a solid to a gas without passing through the liquid phase, typically under low pressure or when heated below its melting point. Common examples include dry ice (solid carbon dioxide) turning directly into carbon dioxide gas and the sublimation of iodine crystals at room temperature. This phase change is critical in applications such as freeze-drying and solid air fresheners.
Real-Life Applications of Freezing
Freezing is commonly used in food preservation to extend shelf life by slowing microbial growth and enzymatic activity, maintaining freshness in products like vegetables, meat, and dairy. Industrial freezing techniques are essential in pharmaceuticals for stabilizing medications and biological samples during storage and transportation. In construction, freezing methods help in soil stabilization and frost heave prevention, ensuring structural integrity in cold climates.
Common Uses of Sublimation in Industry
Sublimation is widely used in industries such as textile printing, where dye sublimation transfers vibrant, durable images onto fabrics. It is essential in manufacturing personalized promotional products like mugs, phone cases, and apparel due to its ability to produce high-resolution, long-lasting designs. Unlike freezing, which is primarily used for preservation, sublimation plays a critical role in creating high-quality, customized consumer goods and industrial components.
Comparing Energy Changes in Freezing vs Sublimation
Freezing involves the release of latent heat as a substance transitions from liquid to solid, typically releasing about 333.55 kJ/kg for water. Sublimation requires a significantly higher energy change since it bypasses the liquid phase, with water needing approximately 2834 kJ/kg to convert directly from solid to gas. This stark difference highlights sublimation's greater energy demand due to overcoming both fusion and vaporization energy barriers.
Summary: Choosing Between Freezing and Sublimation
Freezing preserves materials by lowering their temperature to solidify without changing their chemical structure, ideal for long-term storage of perishable goods such as food and biological samples. Sublimation removes moisture by converting ice directly into vapor under low pressure, making it optimal for delicate items like pharmaceuticals and freeze-dried foods because it minimizes structural damage. Selecting between freezing and sublimation depends on factors like the sensitivity of the product, desired shelf life, preservation quality, and cost-efficiency.
Freezing Infographic
 libterm.com
libterm.com