Chemical processes play a crucial role in manufacturing, environmental management, and pharmaceuticals by transforming raw materials into valuable products through reactions and interactions. Understanding these processes enhances your ability to innovate and improve efficiency within various industries. Explore the rest of the article to uncover detailed applications and benefits of chemical technologies.
Table of Comparison
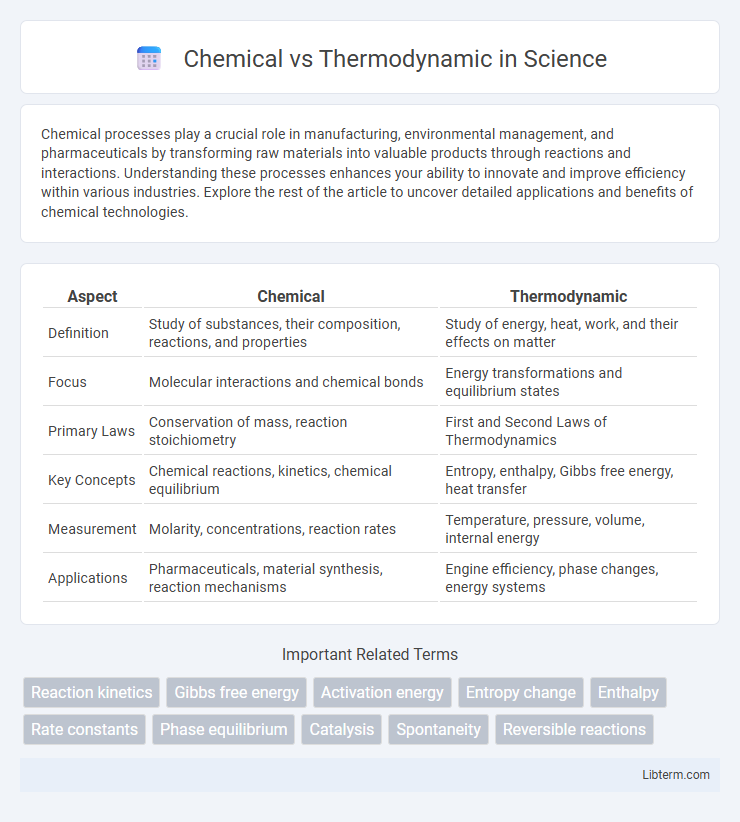
| Aspect | Chemical | Thermodynamic |
|---|---|---|
| Definition | Study of substances, their composition, reactions, and properties | Study of energy, heat, work, and their effects on matter |
| Focus | Molecular interactions and chemical bonds | Energy transformations and equilibrium states |
| Primary Laws | Conservation of mass, reaction stoichiometry | First and Second Laws of Thermodynamics |
| Key Concepts | Chemical reactions, kinetics, chemical equilibrium | Entropy, enthalpy, Gibbs free energy, heat transfer |
| Measurement | Molarity, concentrations, reaction rates | Temperature, pressure, volume, internal energy |
| Applications | Pharmaceuticals, material synthesis, reaction mechanisms | Engine efficiency, phase changes, energy systems |
Introduction to Chemical and Thermodynamic Processes
Chemical processes involve reactions where substances transform through breaking and forming chemical bonds, governed by reaction kinetics and stoichiometry. Thermodynamic processes focus on energy transformations and the laws of thermodynamics, analyzing systems in terms of enthalpy, entropy, and free energy without necessarily involving chemical changes. Understanding both chemical kinetics and thermodynamic principles is essential for optimizing reactions and energy efficiency in industrial applications.
Defining Chemical Changes
Chemical changes involve alterations in the molecular or atomic composition of a substance, resulting in the formation of new chemical species with different properties. Thermodynamic changes relate to the energy transformations and states of matter during these processes, often characterized by changes in enthalpy, entropy, and Gibbs free energy. Defining chemical changes requires recognizing the breaking and formation of chemical bonds, which distinguishes them from mere physical or thermodynamic changes.
Understanding Thermodynamic Principles
Thermodynamic principles govern the energy changes and equilibria in chemical reactions, providing insights into spontaneity and system stability beyond mere chemical mechanisms. Understanding Gibbs free energy, enthalpy, and entropy enables precise prediction of reaction feasibility and direction under varying conditions. This thermodynamic framework complements chemical kinetics by quantifying the balance between energy transformations and molecular interactions.
Key Differences Between Chemical and Thermodynamic Processes
Chemical processes involve changes in molecular composition and chemical bonds, resulting in the formation of new substances, while thermodynamic processes relate to energy changes and transformations within a system, often without altering chemical identity. Chemical reactions are governed by reaction kinetics and mechanisms, whereas thermodynamic processes depend on principles such as enthalpy, entropy, and Gibbs free energy to determine spontaneity and equilibrium. These fundamental differences highlight that chemical processes focus on substance change, whereas thermodynamic processes emphasize energy flow and system properties.
Energy Transformations in Chemical Reactions
Energy transformations in chemical reactions involve the conversion of chemical potential energy into other energy forms, governed by thermodynamic principles such as enthalpy, entropy, and Gibbs free energy. Chemical energy changes during bond breaking and formation determine whether reactions are exothermic or endothermic, influencing spontaneity based on the reaction's thermodynamic parameters. Thermodynamics quantifies the direction and extent of these energy transformations, ensuring that reactions proceed towards a state of minimum free energy for stability.
Thermodynamic Laws in Chemical Systems
Thermodynamic laws govern energy transformations and equilibrium states in chemical systems, ensuring accurate predictions of reaction spontaneity and feasibility. The first law, conservation of energy, relates enthalpy changes during chemical reactions, while the second law introduces entropy, dictating the direction of spontaneous processes. Chemical thermodynamics integrates these principles to optimize reaction conditions and understand phase behavior in complex chemical systems.
Practical Examples: Chemical vs Thermodynamic Applications
Chemical applications include battery design, pharmaceuticals, and catalyst development where reaction rates and molecular interactions are crucial. Thermodynamic applications focus on engine efficiency, refrigeration cycles, and phase change materials, optimizing energy transfer and equilibrium states. Both fields intersect in fuel cells, combining chemical reactions with thermodynamic principles for practical energy solutions.
Measurement Techniques: Chemical Changes vs Thermodynamic Quantities
Chemical changes are analyzed using techniques like titration, spectroscopy, and chromatography to measure reaction progress, concentration changes, and product formation. Thermodynamic quantities such as enthalpy, entropy, and Gibbs free energy are determined through calorimetry, pressure-volume work measurements, and temperature control experiments to quantify energy changes and spontaneity. Accurate assessment of chemical changes relies on detecting molecular transformations, whereas thermodynamic measurements require monitoring energy exchange and state functions under controlled conditions.
Advantages and Limitations of Each Approach
Chemical analysis offers precise identification of substances and their composition, enabling targeted manipulation of molecular structures for specific applications, though it often requires complex instrumentation and may involve hazardous reagents. Thermodynamic methods provide insights into energy transformations, equilibrium states, and system spontaneity, facilitating predictions about reaction feasibility under varying conditions, but they rely heavily on idealized models and assumptions that may not capture real-world complexities. Both approaches complement each other; chemical techniques deliver detailed compositional data while thermodynamics frame the broader energetic context, yet their limitations necessitate integrated use for comprehensive system understanding.
Conclusion: Choosing Between Chemical and Thermodynamic Perspectives
Choosing between chemical and thermodynamic perspectives depends on the specific goals of analysis: chemical approaches emphasize reaction mechanisms, species interactions, and molecular changes, while thermodynamic methods focus on energy profiles, equilibrium states, and system spontaneity. For detailed understanding of reaction pathways and intermediate species, chemical perspective provides essential insights; thermodynamic perspective offers critical predictions about feasibility and stability based on Gibbs free energy and enthalpy values. Integrating both viewpoints yields comprehensive analysis, enabling accurate prediction of chemical behavior and optimization of reaction conditions.
Chemical Infographic
 libterm.com
libterm.com