Rust is a systems programming language focused on safety, performance, and concurrency, making it ideal for developing reliable and efficient software. It eliminates common bugs through strict compile-time checks and offers powerful features like ownership and borrowing to manage memory without a garbage collector. Explore the rest of the article to unlock how Rust can transform your development projects.
Table of Comparison
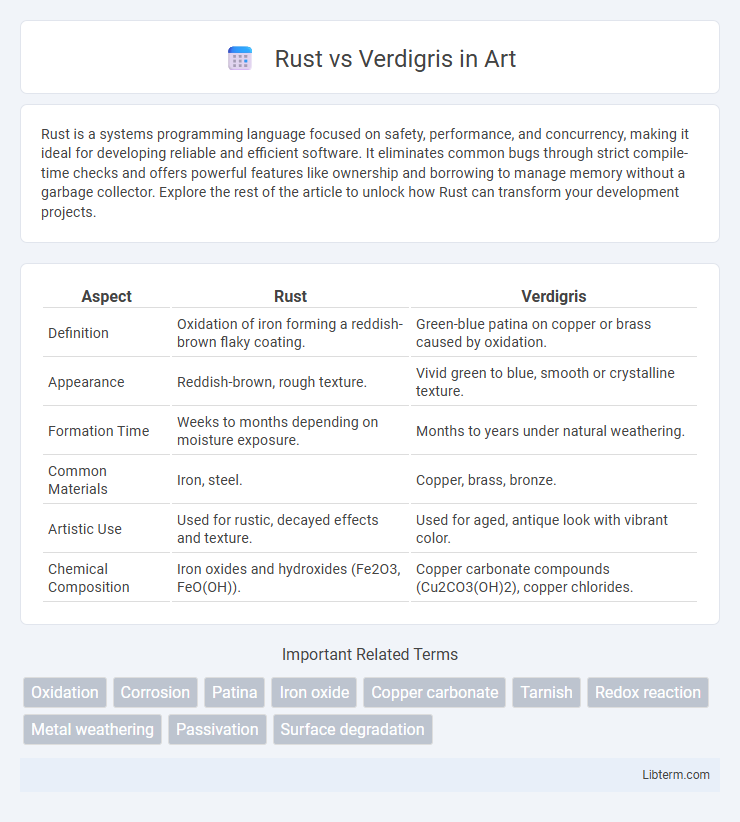
| Aspect | Rust | Verdigris |
|---|---|---|
| Definition | Oxidation of iron forming a reddish-brown flaky coating. | Green-blue patina on copper or brass caused by oxidation. |
| Appearance | Reddish-brown, rough texture. | Vivid green to blue, smooth or crystalline texture. |
| Formation Time | Weeks to months depending on moisture exposure. | Months to years under natural weathering. |
| Common Materials | Iron, steel. | Copper, brass, bronze. |
| Artistic Use | Used for rustic, decayed effects and texture. | Used for aged, antique look with vibrant color. |
| Chemical Composition | Iron oxides and hydroxides (Fe2O3, FeO(OH)). | Copper carbonate compounds (Cu2CO3(OH)2), copper chlorides. |
Introduction to Rust and Verdigris
Rust is a chemical compound formed by the oxidation of iron in the presence of moisture and oxygen, resulting in a reddish-brown flaky coating that weakens metal structures. Verdigris is a green patina primarily composed of copper carbonate that forms on copper, brass, or bronze surfaces due to prolonged exposure to atmospheric elements like carbon dioxide and moisture. Both rust and verdigris signify corrosion but affect different metals and exhibit distinct colors and chemical compositions.
Chemical Composition and Formation
Rust primarily consists of iron oxides, including Fe2O3 and Fe3O4, formed through the oxidation of iron in the presence of oxygen and moisture. Verdigris is mainly composed of copper(II) acetate and other copper salts, produced by the chemical reaction of copper, acetic acid, and oxygen. The electrochemical processes driving rust involve iron's oxidation, whereas verdigris results from copper's corrosion influenced by organic acids.
Causes and Environmental Factors
Rust forms primarily due to the oxidation of iron in the presence of moisture and oxygen, accelerated by factors such as saltwater exposure and acidic rain. Verdigris develops from the corrosion of copper, bronze, or brass when exposed to air, moisture, and acidic conditions, often influenced by pollutants like sulfur dioxide. Both processes are influenced by environmental humidity, temperature fluctuations, and chemical contaminants, which accelerate metal degradation and oxide formation.
Physical Appearance: How to Distinguish Rust and Verdigris
Rust appears as a reddish-brown flaky coating primarily on iron and steel due to oxidation, characterized by its rough texture and brittle nature. Verdigris manifests as a bluish-green patina often found on copper, brass, and bronze surfaces, exhibiting a smooth, sometimes crystalline appearance resulting from copper carbonate formation. Identifying the metal substrate and observing the distinct coloration and texture differences enable accurate differentiation between rust and verdigris.
Effects on Different Materials
Rust primarily affects ferrous metals by causing oxidation that leads to structural weakening and surface deterioration, while verdigris forms on copper and its alloys through a chemical reaction with moisture and air, resulting in a greenish patina that can either protect or degrade the metal. Rust drastically compromises iron and steel, often requiring removal to prevent further damage, whereas verdigris, common on bronze and brass, can provide corrosion resistance but may also cause pitting if left unchecked. Understanding these distinct effects is crucial for material conservation and selecting appropriate prevention or treatment methods.
Impact on Structural Integrity
Rust significantly diminishes structural integrity by causing iron and steel to weaken through oxidation, leading to brittleness and potential failure under stress. Verdigris forms primarily on copper and its alloys, creating a protective patina that slows further corrosion while minimally affecting the metal's strength. The contrasting effects highlight rust as a destructive process undermining load-bearing capacity, whereas verdigris often serves as a protective layer preserving structural stability.
Prevention and Protection Methods
Rust prevention relies on methods such as applying protective coatings like paint, galvanization with zinc, and using corrosion inhibitors to create barriers against moisture and oxygen. Verdigris, the green patina on copper and its alloys, is often controlled by applying lacquers or sealants to prevent exposure to air and acidic conditions that accelerate corrosion. Employing regular maintenance, choosing weather-resistant materials, and ensuring proper surface treatments are essential strategies to enhance the longevity and protective qualities of metals against both rust and verdigris.
Removal Techniques and Best Practices
Rust removal techniques include mechanical abrasion with wire brushes, chemical rust removers containing phosphoric or oxalic acid, and electrolysis for heavy corrosion. Verdigris is commonly removed using mild acids like diluted vinegar or lemon juice, combined with gentle scrubbing to avoid damaging copper or brass surfaces. Best practices involve protecting cleaned metal with rust inhibitors or clear coatings to prevent re-oxidation and using appropriate personal protective equipment during chemical treatments.
Common Uses and Misconceptions
Rust commonly forms on iron and steel surfaces exposed to moisture and oxygen, leading to structural weakening in buildings, bridges, and vehicles, whereas verdigris develops on copper, brass, and bronze as a greenish patina often valued for artistic and architectural aesthetics. Rust is frequently mistaken as merely a cosmetic issue, but it significantly compromises metal integrity and safety, while verdigris is often misunderstood as pure corrosion, despite its role in protecting the underlying metal from further decay. Both substances serve distinct functions and perceptions in metal maintenance and preservation, with rust necessitating removal or treatment and verdigris sometimes preserved for its decorative and protective qualities.
Conclusion: Choosing the Right Solution
Choosing between Rust and Verdigris depends on project requirements and development goals. Rust offers memory safety and concurrency features ideal for performance-critical applications, while Verdigris excels in network programming and asynchronous tasks. Evaluating specific use cases ensures the selection of the most efficient and maintainable solution.
Rust Infographic
 libterm.com
libterm.com