Plating enhances both the appearance and durability of metal products by applying a thin layer of material onto their surfaces. This process improves corrosion resistance, electrical conductivity, and wear protection, making it essential in industries like automotive, electronics, and jewelry. Discover how plating techniques can optimize your projects by reading the full article.
Table of Comparison
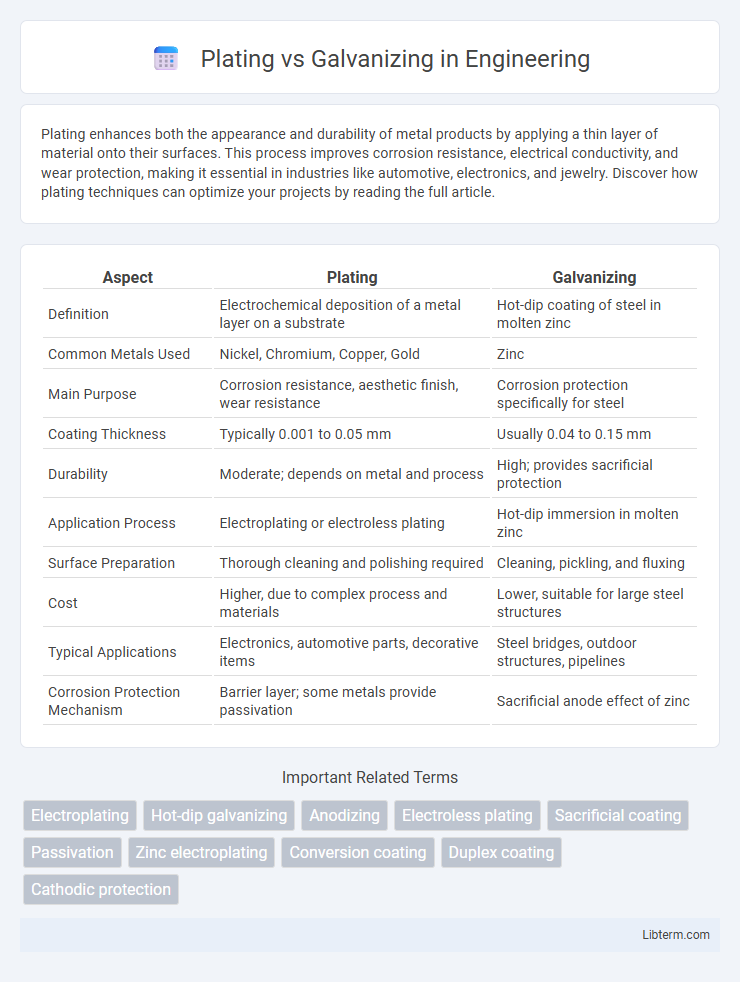
| Aspect | Plating | Galvanizing |
|---|---|---|
| Definition | Electrochemical deposition of a metal layer on a substrate | Hot-dip coating of steel in molten zinc |
| Common Metals Used | Nickel, Chromium, Copper, Gold | Zinc |
| Main Purpose | Corrosion resistance, aesthetic finish, wear resistance | Corrosion protection specifically for steel |
| Coating Thickness | Typically 0.001 to 0.05 mm | Usually 0.04 to 0.15 mm |
| Durability | Moderate; depends on metal and process | High; provides sacrificial protection |
| Application Process | Electroplating or electroless plating | Hot-dip immersion in molten zinc |
| Surface Preparation | Thorough cleaning and polishing required | Cleaning, pickling, and fluxing |
| Cost | Higher, due to complex process and materials | Lower, suitable for large steel structures |
| Typical Applications | Electronics, automotive parts, decorative items | Steel bridges, outdoor structures, pipelines |
| Corrosion Protection Mechanism | Barrier layer; some metals provide passivation | Sacrificial anode effect of zinc |
Introduction to Plating and Galvanizing
Plating involves depositing a thin layer of metal onto a surface to enhance corrosion resistance, improve appearance, or increase wear resistance, commonly using metals like nickel, chrome, or gold. Galvanizing primarily refers to applying a zinc coating to steel or iron through hot-dip or electro-galvanizing processes, providing robust protection against rust and prolonging the base metal's lifespan. Both techniques serve critical roles in industrial applications, with plating offering decorative and functional finishes while galvanizing focuses on preventing corrosion in structural materials.
What is Plating?
Plating is a process that involves depositing a thin layer of metal onto the surface of an object to enhance its appearance, corrosion resistance, or wear resistance. Common plating materials include chrome, nickel, and gold, each chosen for specific protective or decorative properties. This technique is widely used in automotive, electronics, and jewelry industries to improve product durability and aesthetic appeal.
What is Galvanizing?
Galvanizing is a process of applying a protective zinc coating to steel or iron to prevent rusting and corrosion. This method typically involves hot-dip galvanizing, where the metal is submerged in molten zinc, creating a durable, sacrificial layer that protects the underlying metal from environmental damage. Galvanized steel is widely used in construction, automotive, and outdoor applications due to its enhanced longevity and resistance to wear and moisture.
Types of Plating Methods
Electroplating is the most common type of plating method, using electric current to deposit a metal coating, such as nickel or chromium, onto a substrate. Electroless plating offers uniform coatings without electricity by using a chemical reduction process, commonly applied with metals like nickel-phosphorus. Other plating methods include immersion plating, where a metal layer forms through displacement reactions, and mechanical plating, which involves kinetic energy to bond metal particles to surfaces, enhancing corrosion resistance and durability.
Types of Galvanizing Processes
Hot-dip galvanizing, electro-galvanizing, and sherardizing are primary types of galvanizing processes used for corrosion protection. Hot-dip galvanizing involves immersing steel in molten zinc, creating a thick, durable coating ideal for outdoor applications. Electro-galvanizing uses an electrical current to deposit a thinner zinc layer, offering better surface finish but less corrosion resistance compared to hot-dip methods.
Key Differences Between Plating and Galvanizing
Plating involves depositing a thin layer of metal, such as nickel or chrome, onto a substrate for aesthetic appeal and corrosion resistance, whereas galvanizing coats steel or iron with a thick layer of zinc primarily for heavy-duty rust protection. Plating generally provides a smoother, more decorative finish while galvanizing offers enhanced durability and sacrificial protection against corrosion. The plating process is often used in electronics and jewelry, while galvanizing is favored in construction and industrial applications for long-term metal preservation.
Advantages of Plating
Plating offers superior precision in coating thickness control, enabling enhanced protection and aesthetic appeal on delicate or intricate components. It provides excellent corrosion resistance and improved surface hardness, extending the lifespan of metals in various industrial applications. The versatility of plating processes allows for a wide range of metal finishes, including chromium, nickel, and gold, tailored to specific functional and decorative requirements.
Benefits of Galvanizing
Galvanizing offers superior corrosion resistance by forming a durable zinc coating that protects steel from rust and environmental damage. The process provides long-lasting protection with minimal maintenance, making it ideal for outdoor and industrial applications. Galvanized steel also delivers excellent abrasion resistance and cost-effectiveness due to its extended lifespan and reduced need for repairs.
Common Applications: Plating vs Galvanizing
Plating is commonly used in electronics, automotive parts, and decorative items to enhance corrosion resistance and aesthetic appeal with thin metal coatings such as chrome or nickel. Galvanizing is widely applied in construction, infrastructure, and outdoor equipment, where steel components receive a thick zinc coating for robust protection against rust and environmental exposure. Both processes serve to protect metal surfaces but vary significantly in coating thickness and durability tailored to their specific industrial applications.
Choosing the Right Protection Method
Selecting the appropriate protection method between plating and galvanizing depends on factors such as environmental exposure, desired durability, and cost-effectiveness. Plating offers superior aesthetic finishes and corrosion resistance for indoor applications, while galvanizing provides a robust, long-lasting zinc coating ideal for outdoor and industrial conditions. Understanding the specific requirements of the project, including lifespan expectations and maintenance needs, ensures optimal metal protection and performance.
Plating Infographic
 libterm.com
libterm.com