Hemostasis is the physiological process that stops bleeding by clot formation to prevent excessive blood loss after vascular injury. It involves a complex interplay between blood vessels, platelets, and coagulation factors to form a stable blood clot. Discover how understanding hemostasis can benefit your knowledge of wound healing and bleeding disorders in the rest of this article.
Table of Comparison
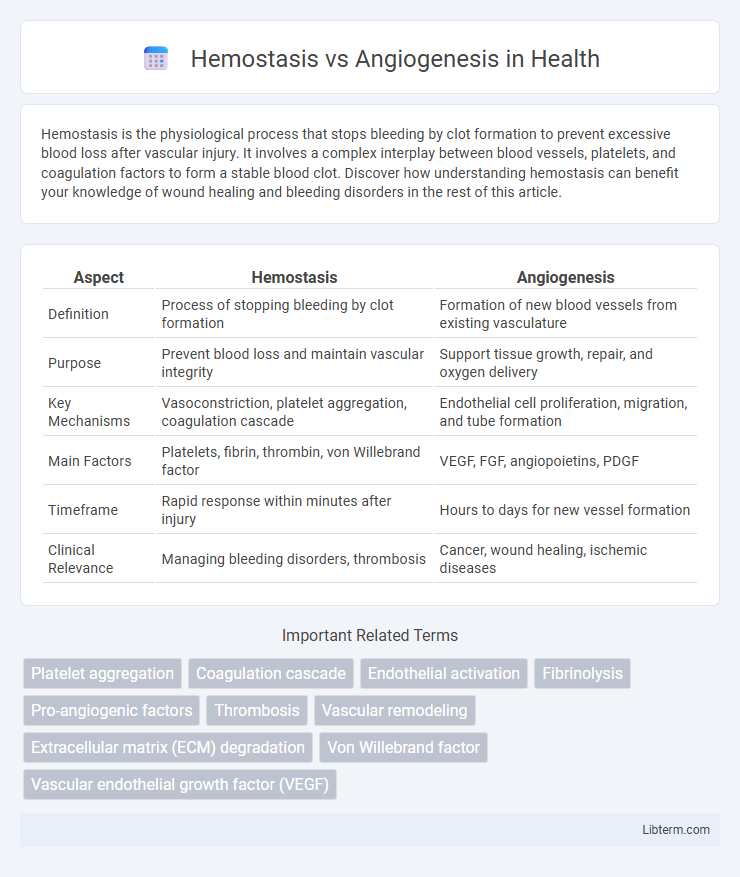
| Aspect | Hemostasis | Angiogenesis |
|---|---|---|
| Definition | Process of stopping bleeding by clot formation | Formation of new blood vessels from existing vasculature |
| Purpose | Prevent blood loss and maintain vascular integrity | Support tissue growth, repair, and oxygen delivery |
| Key Mechanisms | Vasoconstriction, platelet aggregation, coagulation cascade | Endothelial cell proliferation, migration, and tube formation |
| Main Factors | Platelets, fibrin, thrombin, von Willebrand factor | VEGF, FGF, angiopoietins, PDGF |
| Timeframe | Rapid response within minutes after injury | Hours to days for new vessel formation |
| Clinical Relevance | Managing bleeding disorders, thrombosis | Cancer, wound healing, ischemic diseases |
Introduction to Hemostasis and Angiogenesis
Hemostasis is the physiological process that prevents excessive bleeding by initiating blood clot formation at injury sites through vascular constriction, platelet aggregation, and coagulation cascades. Angiogenesis involves the growth of new blood vessels from pre-existing vasculature, critical for tissue repair, wound healing, and pathologies like cancer. Both processes are tightly regulated mechanisms essential for maintaining vascular integrity and promoting tissue regeneration.
Defining Hemostasis: Key Mechanisms
Hemostasis is a crucial physiological process that prevents excessive bleeding by forming blood clots through vascular constriction, platelet aggregation, and the coagulation cascade. Key mechanisms include primary hemostasis, where platelets adhere to the damaged endothelium, and secondary hemostasis, involving the activation of clotting factors that convert fibrinogen to fibrin to stabilize the clot. This tightly regulated sequence contrasts with angiogenesis, which promotes new blood vessel formation essential for tissue repair and growth rather than immediate bleeding control.
Understanding Angiogenesis: The Basics
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, essential for growth, wound healing, and tissue regeneration. It involves the activation, proliferation, and migration of endothelial cells in response to signaling molecules such as vascular endothelial growth factor (VEGF). Unlike hemostasis, which stops bleeding and maintains vascular integrity, angiogenesis facilitates oxygen and nutrient delivery to tissues, playing a critical role in both normal development and pathological conditions such as cancer and diabetic retinopathy.
Cellular Players in Hemostasis vs. Angiogenesis
Platelets and endothelial cells are the primary cellular players in hemostasis, where platelets aggregate to form a clot while endothelial cells release clotting factors to prevent excessive bleeding. In angiogenesis, endothelial cells play a central role by proliferating and migrating to form new blood vessels, supported by pericytes and smooth muscle cells that stabilize the newly formed vasculature. Macrophages contribute to both processes by releasing cytokines that regulate clot formation in hemostasis and by secreting growth factors like VEGF to promote angiogenesis.
Molecular Pathways: Comparing Hemostasis and Angiogenesis
Hemostasis involves molecular pathways such as platelet activation, coagulation cascades including the intrinsic and extrinsic pathways, and fibrin clot formation mediated by thrombin and fibrinogen. Angiogenesis is regulated by signaling pathways like VEGF (vascular endothelial growth factor), angiopoietins, and Notch signaling, which promote endothelial cell proliferation, migration, and new blood vessel formation. Both processes share common molecules like thrombin and integrins but differ fundamentally in their cellular responses and outcomes--hemostasis aims to prevent blood loss through clot stabilization, while angiogenesis facilitates tissue repair and neovascularization.
Hemostasis in Wound Healing
Hemostasis is the critical initial phase of wound healing, involving vasoconstriction and the formation of a platelet plug to prevent excessive blood loss. Platelet activation triggers the coagulation cascade, stabilizing the clot with fibrin mesh and providing a temporary barrier against pathogens. Efficient hemostasis is essential to create a conducive environment for subsequent processes like angiogenesis and tissue regeneration.
Angiogenesis in Tissue Regeneration
Angiogenesis plays a critical role in tissue regeneration by promoting the formation of new blood vessels, which supply oxygen and nutrients essential for healing damaged tissues. Unlike hemostasis, which quickly stops bleeding through clot formation, angiogenesis supports long-term repair through endothelial cell proliferation and migration. Growth factors such as VEGF (vascular endothelial growth factor) are key regulators that stimulate angiogenesis during the regenerative process, enhancing tissue recovery and functional restoration.
Clinical Significance: Hemostasis vs. Angiogenesis
Hemostasis plays a critical role in preventing excessive bleeding during injury by facilitating blood clot formation, essential in managing trauma and surgical outcomes. Angiogenesis is pivotal in wound healing and tissue regeneration, with dysregulated angiogenesis contributing to diseases such as cancer, diabetic retinopathy, and cardiovascular disorders. Therapeutic targeting of hemostatic factors or angiogenic pathways offers promising clinical interventions in controlling bleeding disorders and inhibiting tumor growth, respectively.
Disorders Linked to Hemostasis and Angiogenesis Imbalance
Disorders linked to hemostasis and angiogenesis imbalance include thrombosis, where excessive clot formation disrupts normal blood flow, and cancer, characterized by abnormal angiogenesis facilitating tumor growth and metastasis. Hemophilia exemplifies a hemostasis disorder caused by clotting factor deficiencies, leading to persistent bleeding, while diabetic retinopathy results from pathological angiogenesis damaging retinal blood vessels. Understanding these conditions highlights the critical role of maintaining a balance between coagulation processes and new blood vessel formation for vascular health.
Future Perspectives and Therapeutic Implications
Emerging therapies targeting hemostasis and angiogenesis hold promise for treating cardiovascular and cancer-related diseases by modulating clot formation and new blood vessel growth. Advanced biomaterials and gene editing tools, such as CRISPR-Cas9, offer precise control over these processes, potentially improving wound healing and inhibiting tumor progression. Future research aims to integrate personalized medicine approaches to optimize treatment efficacy and minimize adverse effects in managing vascular disorders.
Hemostasis Infographic
 libterm.com
libterm.com