Bioequivalence ensures that two pharmaceutical products deliver the same therapeutic effect by comparing their bioavailability parameters such as absorption rate and extent. This concept allows generic drugs to be considered interchangeable with branded medications, ensuring safety and efficacy for patients. Explore the full article to understand how bioequivalence impacts your medication choices and treatment outcomes.
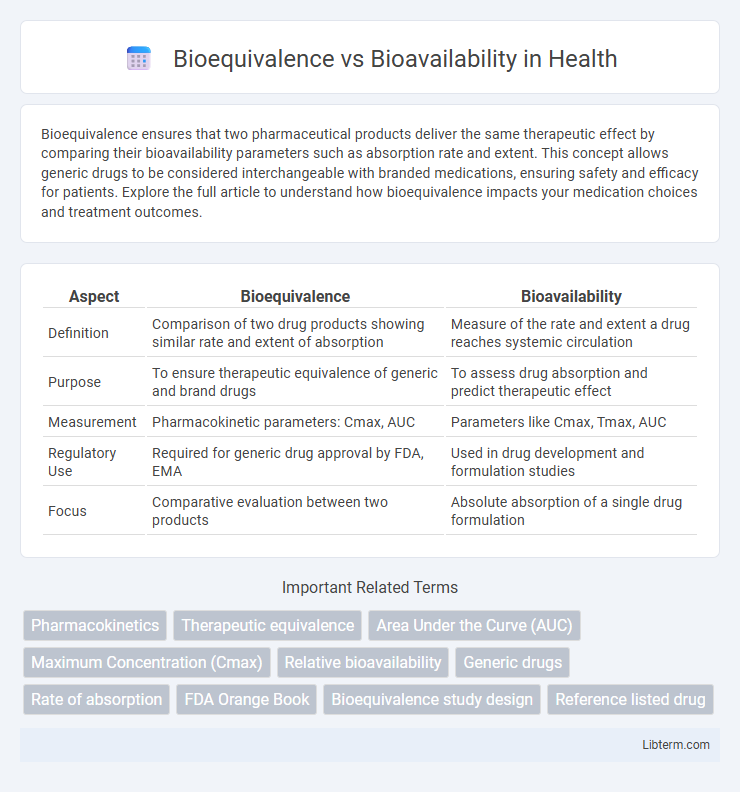
Table of Comparison
| Aspect | Bioequivalence | Bioavailability |
|---|---|---|
| Definition | Comparison of two drug products showing similar rate and extent of absorption | Measure of the rate and extent a drug reaches systemic circulation |
| Purpose | To ensure therapeutic equivalence of generic and brand drugs | To assess drug absorption and predict therapeutic effect |
| Measurement | Pharmacokinetic parameters: Cmax, AUC | Parameters like Cmax, Tmax, AUC |
| Regulatory Use | Required for generic drug approval by FDA, EMA | Used in drug development and formulation studies |
| Focus | Comparative evaluation between two products | Absolute absorption of a single drug formulation |
Introduction to Bioequivalence and Bioavailability
Bioavailability measures the rate and extent to which the active pharmaceutical ingredient is absorbed and becomes available at the site of action. Bioequivalence compares the bioavailability of two drug products, typically a generic and its brand-name counterpart, to ensure therapeutic equivalence. Both parameters are critical in drug development and regulatory approval to guarantee efficacy and safety.
Defining Bioavailability: Key Concepts
Bioavailability refers to the proportion of an administered drug that reaches systemic circulation in an active form, directly impacting therapeutic efficacy. Key concepts include the rate and extent of absorption, influenced by drug formulation, route of administration, and first-pass metabolism. Measuring bioavailability involves pharmacokinetic parameters such as Cmax (maximum concentration) and AUC (area under the concentration-time curve) to assess drug availability in plasma.
Understanding Bioequivalence: Core Principles
Bioequivalence evaluates whether two drug formulations release the active ingredient into the bloodstream at the same rate and extent, ensuring therapeutic equivalence. Key parameters include Cmax (maximum concentration), Tmax (time to reach Cmax), and AUC (area under the concentration-time curve), which quantify the drug's absorption profile. Regulatory agencies require bioequivalence studies to confirm that generic drugs perform similarly to their branded counterparts in terms of efficacy and safety.
Importance in Drug Development and Approval
Bioequivalence and bioavailability are critical metrics in drug development and regulatory approval, ensuring that generic formulations deliver the same therapeutic effect as original branded drugs. Demonstrating bioequivalence involves comparing the rate and extent of absorption of the active pharmaceutical ingredient, which directly influences drug efficacy and safety profiles. Regulatory agencies like the FDA and EMA prioritize bioequivalence studies to approve generic drugs, reducing development costs and accelerating market access while maintaining patient trust and treatment outcomes.
Methods for Measuring Bioavailability
Methods for measuring bioavailability primarily involve pharmacokinetic studies that quantify the rate and extent of drug absorption into systemic circulation. Techniques such as plasma concentration-time profiling, urinary excretion analysis, and the use of biomarkers are standard for assessing how much and how quickly an active pharmaceutical ingredient reaches target sites. Advanced methods like isotopic labeling and non-invasive imaging further enhance accuracy in differentiating bioavailability parameters from bioequivalence studies.
Techniques for Assessing Bioequivalence
Techniques for assessing bioequivalence primarily involve pharmacokinetic studies comparing parameters such as Cmax, Tmax, and AUC between test and reference drug formulations to ensure similar absorption rates and extents. Analytical methods like high-performance liquid chromatography (HPLC) and mass spectrometry quantify drug concentration in plasma, enabling precise evaluation of bioequivalence. Statistical approaches, including 90% confidence interval analysis for the ratio of pharmacokinetic metrics, determine equivalence within regulatory bioequivalence limits, typically 80-125%.
Regulatory Guidelines and Standards
Regulatory guidelines by agencies such as the FDA and EMA distinguish bioequivalence (BE) as the requirement to demonstrate that two drug products release the active ingredient into the bloodstream at the same rate and extent, ensuring therapeutic equivalence. Bioavailability (BA) refers to the rate and extent of absorption of the active pharmaceutical ingredient, directly impacting efficacy and safety profiles, with regulatory standards mandating detailed pharmacokinetic studies including Cmax and AUC parameters. Compliance with strict criteria in these bioequivalence and bioavailability assessments supports generic drug approval and protects public health by ensuring consistent drug performance.
Bioequivalence vs Bioavailability: Key Differences
Bioavailability measures the rate and extent to which an active drug ingredient is absorbed and becomes available at the site of action, while bioequivalence assesses whether two drug products with the same active ingredient have comparable bioavailability and pharmacokinetic profiles. Key differences include bioavailability being a singular parameter evaluating drug absorption in a specific dosage form, whereas bioequivalence compares multiple products to ensure therapeutic equivalence. Regulatory agencies require bioequivalence studies for generic drug approval to confirm similar therapeutic effects without independent bioavailability testing for patented drugs.
Clinical Implications for Patients and Practitioners
Bioequivalence ensures that two drug products release the active ingredient into the bloodstream at the same rate and extent, which is critical for generic drug approval and safe substitution in clinical practice. Bioavailability, the measure of the fraction of an administered dose reaching systemic circulation, influences drug efficacy and dosing regimens, impacting therapeutic outcomes. Understanding both bioequivalence and bioavailability guides clinicians in making informed decisions about medication switches, minimizing adverse effects, and optimizing patient care.
Summary and Future Perspectives
Bioequivalence and bioavailability both assess drug absorption, with bioavailability measuring the extent and rate a drug reaches systemic circulation and bioequivalence comparing the bioavailability between two formulations. Future perspectives emphasize advanced pharmacokinetic modeling and in vitro-in vivo correlation techniques to improve predictive accuracy and regulatory decision-making. Emerging technologies such as nanocarriers and personalized medicine are expected to refine bioequivalence studies, enhancing therapeutic efficacy and safety profiles.
Bioequivalence Infographic
 libterm.com
libterm.com