Gastrinoma is a rare type of neuroendocrine tumor that secretes excessive gastrin, leading to increased stomach acid production and peptic ulcers. Early diagnosis and effective treatment are crucial to manage symptoms and prevent complications such as Zollinger-Ellison syndrome. Learn more about the causes, symptoms, and treatment options for gastrinoma in the rest of the article.
Table of Comparison
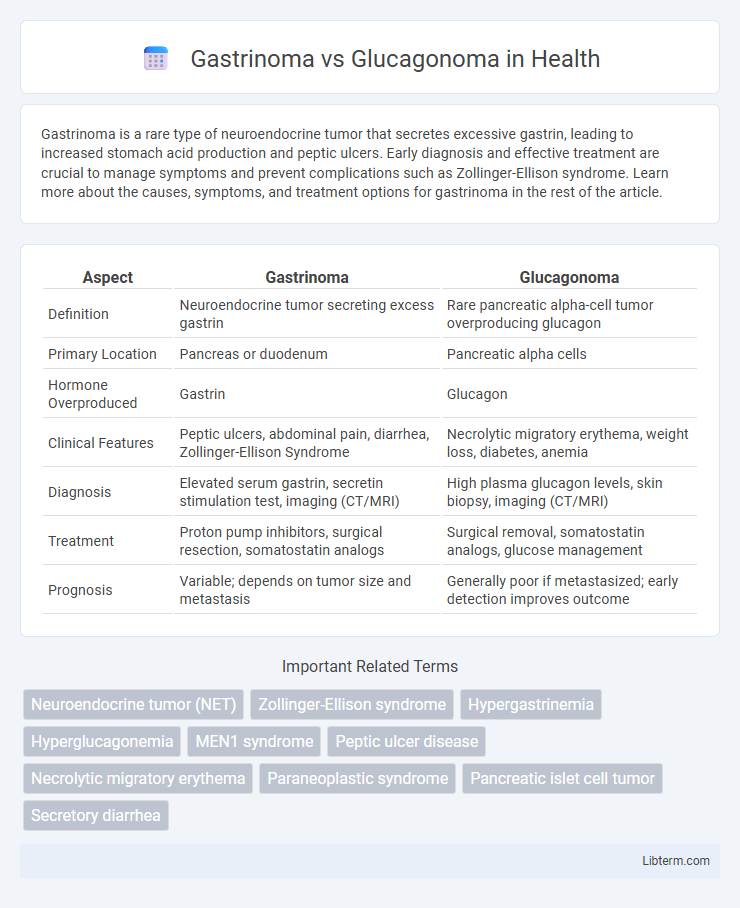
| Aspect | Gastrinoma | Glucagonoma |
|---|---|---|
| Definition | Neuroendocrine tumor secreting excess gastrin | Rare pancreatic alpha-cell tumor overproducing glucagon |
| Primary Location | Pancreas or duodenum | Pancreatic alpha cells |
| Hormone Overproduced | Gastrin | Glucagon |
| Clinical Features | Peptic ulcers, abdominal pain, diarrhea, Zollinger-Ellison Syndrome | Necrolytic migratory erythema, weight loss, diabetes, anemia |
| Diagnosis | Elevated serum gastrin, secretin stimulation test, imaging (CT/MRI) | High plasma glucagon levels, skin biopsy, imaging (CT/MRI) |
| Treatment | Proton pump inhibitors, surgical resection, somatostatin analogs | Surgical removal, somatostatin analogs, glucose management |
| Prognosis | Variable; depends on tumor size and metastasis | Generally poor if metastasized; early detection improves outcome |
Overview of Gastrinoma and Glucagonoma
Gastrinomas are rare neuroendocrine tumors that primarily secrete excess gastrin, leading to Zollinger-Ellison syndrome characterized by severe gastric acid hypersecretion and peptic ulcers. Glucagonomas are neuroendocrine tumors originating from pancreatic alpha cells, causing overproduction of glucagon, which results in necrolytic migratory erythema, diabetes mellitus, and weight loss. Both tumors are malignant with potential for metastasis, but their clinical manifestations and biochemical profiles differ significantly due to the distinct hormones they secrete.
Origin and Pathophysiology
Gastrinomas originate from pancreatic islet cells or duodenal neuroendocrine cells and secrete excessive gastrin, leading to hypergastrinemia and resultant Zollinger-Ellison syndrome characterized by severe gastric acid hypersecretion and recurrent peptic ulcers. Glucagonomas arise from pancreatic alpha cells, causing overproduction of glucagon that induces hyperglycemia, weight loss, and necrolytic migratory erythema due to altered glucose metabolism and amino acid depletion. Both tumors are rare pancreatic neuroendocrine neoplasms but differ significantly in their hormonal profiles and clinical manifestations driven by distinct pathophysiological mechanisms.
Clinical Manifestations
Gastrinoma primarily presents with recurrent peptic ulcers, abdominal pain, and diarrhea due to excessive gastrin secretion causing hypergastrinemia and gastric acid hypersecretion. Glucagonoma is characterized by necrolytic migratory erythema, weight loss, diabetes mellitus, and anemia, reflecting hyperglucagonemia's impact on glucose metabolism and skin integrity. Both neuroendocrine tumors show distinct clinical manifestations linked to their hormone secretion profiles, aiding differential diagnosis.
Hormonal Activity and Effects
Gastrinomas secrete excessive gastrin, causing increased gastric acid production that leads to peptic ulcers and Zollinger-Ellison syndrome, characterized by abdominal pain and diarrhea. Glucagonomas produce excess glucagon, resulting in hyperglycemia, weight loss, and necrolytic migratory erythema, a distinctive skin rash. Both are pancreatic neuroendocrine tumors but differ significantly in hormonal profiles and clinical manifestations due to their distinct secreted hormones.
Diagnostic Criteria and Biomarkers
Gastrinoma diagnosis relies on elevated fasting serum gastrin levels exceeding 1000 pg/mL combined with gastric acid hypersecretion and positive secretin stimulation test results. Glucagonoma is identified by markedly increased plasma glucagon concentrations above 500 pg/mL alongside characteristic clinical features such as necrolytic migratory erythema and diabetes mellitus. Imaging studies like somatostatin receptor scintigraphy and endoscopic ultrasound further support tumor localization in both neuroendocrine tumor types.
Imaging and Localization Techniques
Gastrinomas are primarily localized using somatostatin receptor scintigraphy (SRS) and endoscopic ultrasound (EUS), which effectively detect tumors in the pancreas and duodenum by highlighting somatostatin receptor-expressing cells. Glucagonomas demand multimodal imaging, combining CT scans, MRI, and functional imaging like PET with radiolabeled glucagon analogs to identify typically large pancreatic masses with metastatic disease. Both tumors benefit from selective arterial calcium stimulation with hepatic venous sampling for precise localization of hormone secretion sites in the pancreas.
Differential Diagnosis
Gastrinoma, characterized by excessive gastrin secretion leading to Zollinger-Ellison syndrome, differs from glucagonoma which manifests with hyperglucagonemia causing necrolytic migratory erythema and diabetes mellitus. Differential diagnosis involves measuring fasting gastrin levels and secretin stimulation tests for gastrinoma, while elevated plasma glucagon levels and clinical features like weight loss and stomatitis indicate glucagonoma. Imaging techniques such as somatostatin receptor scintigraphy and endoscopic ultrasound assist in tumor localization for both neuroendocrine tumors.
Treatment Strategies and Management
Gastrinoma treatment primarily involves surgical resection combined with proton pump inhibitors to control gastric acid hypersecretion, while metastatic cases may require somatostatin analogs or targeted therapies like everolimus. Glucagonoma management focuses on tumor removal and symptom control with somatostatin analogs to reduce glucagon secretion, alongside nutritional support for necrolytic migratory erythema and diabetes management. Both conditions benefit from multidisciplinary care including oncologists, endocrinologists, and surgeons to optimize clinical outcomes.
Prognosis and Long-Term Outcomes
Gastrinomas typically have a better prognosis with a 5-year survival rate exceeding 70%, especially when localized and surgically resected early, while metastatic cases significantly reduce survival chances. Glucagonomas often present with higher malignancy rates and poorer long-term outcomes, with 5-year survival rates around 40-50% due to late diagnosis and aggressive tumor behavior. Effective management and early detection remain crucial for improving survival in both neuroendocrine tumors.
Recent Advances and Future Directions
Recent advances in gastrinoma and glucagonoma research include the identification of novel biomarkers such as progastrin-releasing peptide (ProGRP) for gastrinoma and circulating glucagon levels for glucagonoma, enhancing early diagnosis and monitoring. Molecular studies highlight the role of mutations in the MEN1 gene in gastrinoma pathogenesis, while glucagonoma research emphasizes altered glucagon receptor signaling pathways. Future directions involve precision medicine approaches, including targeted therapies against somatostatin receptors and immunotherapies, alongside the development of liquid biopsy techniques for non-invasive tumor profiling and treatment response evaluation.
Gastrinoma Infographic
 libterm.com
libterm.com