Hemostasis is the physiological process that stops bleeding by forming blood clots and repairing damaged blood vessels. It involves a complex interplay between blood platelets, coagulation factors, and the vascular system to maintain the balance between bleeding and clotting. Discover how understanding hemostasis can improve your approach to managing bleeding disorders in the rest of this article.
Table of Comparison
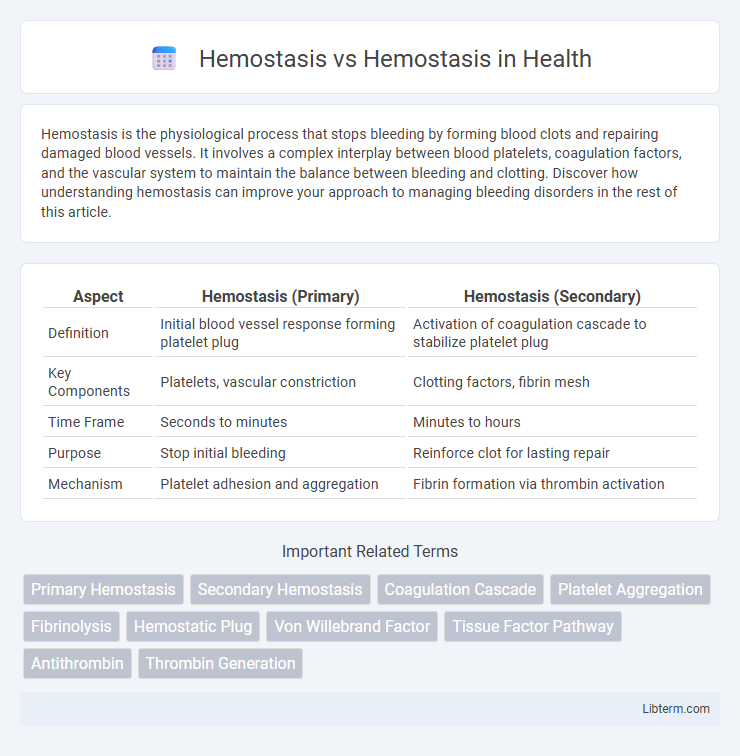
| Aspect | Hemostasis (Primary) | Hemostasis (Secondary) |
|---|---|---|
| Definition | Initial blood vessel response forming platelet plug | Activation of coagulation cascade to stabilize platelet plug |
| Key Components | Platelets, vascular constriction | Clotting factors, fibrin mesh |
| Time Frame | Seconds to minutes | Minutes to hours |
| Purpose | Stop initial bleeding | Reinforce clot for lasting repair |
| Mechanism | Platelet adhesion and aggregation | Fibrin formation via thrombin activation |
Understanding Hemostasis: Definition and Importance
Hemostasis is the physiological process that stops bleeding by forming blood clots through vascular constriction, platelet aggregation, and coagulation cascade activation. It is critical for maintaining vascular integrity and preventing excessive blood loss during injury. Effective hemostasis balances clot formation and dissolution to avoid disorders such as hemorrhage or thrombosis.
Hemostasis vs. Hemostasis: Clarifying the Confusion
Hemostasis refers to the physiological process that stops bleeding by clot formation, whereas homeostasis involves the body's overall maintenance of stable internal conditions such as temperature, pH, and electrolyte balance. Confusion often arises due to the similar spelling and pronunciation, but hemostasis is a specific biological mechanism critical in wound healing and vascular repair. Understanding their distinct roles highlights that hemostasis is a subset of the broader concept of homeostasis within human physiology.
Components of Hemostasis: Key Players
The components of hemostasis include platelets, the vascular endothelium, and coagulation factors, which work together to prevent bleeding and maintain blood vessel integrity. Platelets initiate clot formation by adhering to the damaged endothelium and releasing granules that activate coagulation pathways. Coagulation factors, primarily produced in the liver, sequentially activate to form fibrin, stabilizing the platelet plug and ensuring effective hemostatic response.
Primary vs. Secondary Hemostasis: Key Differences
Primary hemostasis involves the rapid formation of a platelet plug at the site of vascular injury, initiated by platelet adhesion, activation, and aggregation. Secondary hemostasis stabilizes this platelet plug through the activation of the coagulation cascade, leading to fibrin mesh formation that reinforces the clot. The key differences lie in the cellular platelet activity of primary hemostasis versus the enzymatic clotting factor-driven fibrin generation in secondary hemostasis.
Stages of Hemostasis: Step-by-Step Overview
Hemostasis involves three critical stages: vascular spasm, platelet plug formation, and coagulation. During vascular spasm, blood vessels constrict to reduce blood flow; platelet plug formation follows as platelets adhere to the injury site, creating a temporary seal. The coagulation stage stabilizes the plug via a cascade of clotting factors producing fibrin strands, solidifying the clot and preventing excessive bleeding.
Clinical Relevance: Hemostasis in Medical Practice
Hemostasis is crucial in medical practice for controlling bleeding during surgeries and trauma, preventing excessive blood loss and facilitating wound healing. Understanding the balance between coagulation and fibrinolysis helps clinicians manage conditions like hemophilia, thrombosis, and disseminated intravascular coagulation (DIC). Effective hemostatic management also involves the use of anticoagulants, platelet transfusions, and hemostatic agents to achieve optimal patient outcomes.
Disorders Affecting Hemostasis: Risks and Complications
Disorders affecting hemostasis include bleeding disorders such as hemophilia and von Willebrand disease, which impair clot formation and increase bleeding risks. Thrombophilia, characterized by an abnormal tendency to form clots, can lead to complications like deep vein thrombosis and pulmonary embolism. These conditions disrupt the delicate balance of coagulation and fibrinolysis, elevating risks of severe hemorrhage or pathological thrombosis.
Hemostatic Agents: Types and Mechanisms
Hemostatic agents are classified into mechanical, topical, and pharmacologic types based on their mechanisms of action in controlling bleeding. Mechanical agents provide physical barriers or pressure to stem blood flow, while topical agents promote clot formation through activated substances like thrombin or fibrin. Pharmacologic agents act systemically by enhancing the coagulation cascade or inhibiting fibrinolysis, effectively stabilizing hemostasis during surgical and trauma interventions.
Diagnostic Approaches in Hemostasis Evaluation
Diagnostic approaches in hemostasis evaluation primarily involve coagulation assays such as prothrombin time (PT), activated partial thromboplastin time (aPTT), and thrombin time (TT) to assess the intrinsic, extrinsic, and common pathways of the coagulation cascade. Platelet function tests, including platelet aggregation studies and bleeding time, help evaluate primary hemostasis by analyzing platelet plug formation and interaction with blood vessel walls. Advanced diagnostics like thromboelastography (TEG) provide real-time monitoring of clot formation, strength, and dissolution, offering comprehensive hemostatic profiling in clinical settings.
Future Advances in Hemostasis Research
Future advances in hemostasis research emphasize the development of targeted therapies that enhance clot stability while minimizing thrombotic risks. Innovations in bioengineering offer new hemostatic agents, including nanoparticle-based drug delivery systems that improve precision and efficacy in controlling bleeding. Integrating genomics and proteomics paves the way for personalized hemostatic treatments tailored to individual coagulation profiles, optimizing patient outcomes in both surgical and trauma settings.
Hemostasis Infographic
 libterm.com
libterm.com