Hyperosmotic solutions contain a higher concentration of solutes compared to the surrounding environment, causing water to move out of cells by osmosis. This process is critical in medical treatments like peritoneal dialysis and can affect cellular hydration levels drastically. Discover how hyperosmotic effects impact your body and treatments by reading the full article.
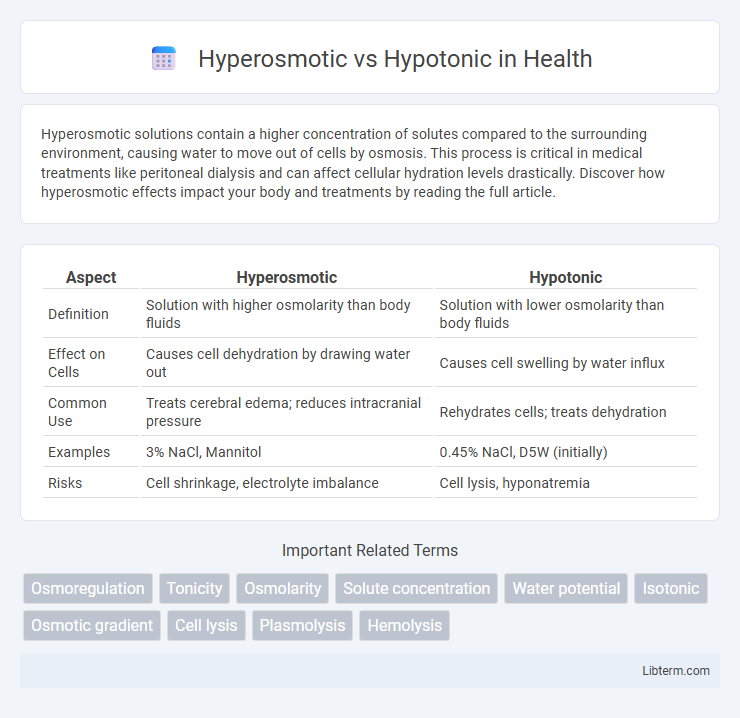
Table of Comparison
| Aspect | Hyperosmotic | Hypotonic |
|---|---|---|
| Definition | Solution with higher osmolarity than body fluids | Solution with lower osmolarity than body fluids |
| Effect on Cells | Causes cell dehydration by drawing water out | Causes cell swelling by water influx |
| Common Use | Treats cerebral edema; reduces intracranial pressure | Rehydrates cells; treats dehydration |
| Examples | 3% NaCl, Mannitol | 0.45% NaCl, D5W (initially) |
| Risks | Cell shrinkage, electrolyte imbalance | Cell lysis, hyponatremia |
Introduction to Hyperosmotic and Hypotonic Solutions
Hyperosmotic solutions have a higher solute concentration compared to the intracellular fluid, causing water to move out of cells, leading to cell shrinkage. Hypotonic solutions contain a lower solute concentration than the intracellular fluid, resulting in water influx and cell swelling. Understanding these differences is critical in medical treatments involving fluid balance and cellular hydration.
Definition of Hyperosmotic Solutions
Hyperosmotic solutions contain a higher concentration of solutes compared to the cytoplasm of the cell, causing water to move out of the cell and leading to cell shrinkage. These solutions have an osmolarity greater than that of the intracellular fluid, often exceeding 300 mOsm/L. In contrast, hypotonic solutions have a lower solute concentration, resulting in water entering the cell and potential swelling.
Definition of Hypotonic Solutions
Hypotonic solutions have a lower concentration of solutes compared to the interior of a cell, causing water to move into the cell by osmosis. This influx of water can lead to cell swelling or even lysis in extreme cases. In contrast, hyperosmotic solutions contain a higher solute concentration than the cell, drawing water out and causing cell shrinkage.
Osmosis: Key Principles and Mechanisms
Hyperosmotic solutions have a higher solute concentration than the cell's cytoplasm, causing water to move out of the cell via osmosis, leading to cell shrinkage; hypotonic solutions contain lower solute concentrations, resulting in water influx that can cause cell swelling or lysis. Osmosis relies on the semi-permeable membrane's selective permeability and the chemical potential gradient driven by solute concentration differences across the membrane. The key mechanisms involve the passive diffusion of water molecules from regions of low solute concentration to high solute concentration to achieve osmotic equilibrium.
Hyperosmotic vs Hypotonic: Core Differences
Hyperosmotic solutions have a higher solute concentration compared to the inside of a cell, causing water to move out and leading to cell shrinkage, whereas hypotonic solutions have a lower solute concentration, resulting in water influx and cell swelling. Hyperosmotic environments increase osmotic pressure significantly, which affects cellular volume and function more aggressively than hypotonic environments. Understanding these core differences is crucial in medical treatments like intravenous fluid administration and cell preservation techniques.
Biological Impacts on Cells
Hyperosmotic environments cause water to move out of cells, leading to cell shrinkage and potential disruption of cellular metabolism. Hypotonic conditions drive water into cells, causing swelling and possible lysis due to osmotic pressure imbalance. Both conditions critically affect cell volume regulation, membrane integrity, and overall physiological function in biological systems.
Clinical Applications and Relevance
Hyperosmotic solutions, such as mannitol or hypertonic saline, are used clinically to reduce cerebral edema by drawing water out of brain tissue into the bloodstream, effectively decreasing intracranial pressure in conditions like traumatic brain injury or stroke. Hypotonic solutions, including 0.45% saline, are applied to treat cellular dehydration by promoting water movement into cells, commonly used in hypernatremia management or diabetic ketoacidosis correction. Understanding the osmolarity differences aids in precise fluid therapy to avoid complications like cellular swelling or shrinkage, critical in critical care and nephrology settings.
Examples in Everyday Life
Hyperosmotic solutions, such as seawater or concentrated saline eye drops, have a higher solute concentration than the cells they interact with, causing water to move out of cells, leading to shrinkage. Hypotonic solutions like distilled water or dilute salt solutions have lower solute concentrations than cells, resulting in water influx that can cause cells to swell or burst. Everyday examples include using hyperosmotic saline nasal sprays to reduce nasal congestion and hypotonic intravenous fluids like 0.45% saline to rehydrate patients gently.
Diagnostic and Experimental Use
Hyperosmotic solutions, with higher solute concentration than cell cytoplasm, are used experimentally to induce cell shrinkage for studying osmoregulation and membrane permeability. Hypotonic solutions, having lower solute concentration, cause cell swelling and are utilized diagnostically to assess cell membrane integrity and osmotic fragility, especially in red blood cells. Both solutions serve critical roles in experiments probing cellular responses to osmotic stress and in diagnostic assays detecting hemolytic anemia.
Summary and Key Takeaways
Hyperosmotic solutions have a higher solute concentration than the cell's cytoplasm, causing water to move out of the cell and leading to cell shrinkage or crenation. Hypotonic solutions contain a lower solute concentration than the cell, resulting in water influx, swelling, and potential cell lysis. Understanding osmotic gradients is crucial for applications in medical treatments, cell culture, and fluid balance management.
Hyperosmotic Infographic
 libterm.com
libterm.com