Bone marrow failure occurs when the bone marrow is unable to produce sufficient blood cells, leading to conditions like anemia, infection risk, and bleeding problems. This serious disorder can result from genetic factors, exposure to toxic chemicals, certain medications, or autoimmune diseases. Learn more about symptoms, causes, and treatment options to manage your health effectively in the rest of this article.
Table of Comparison
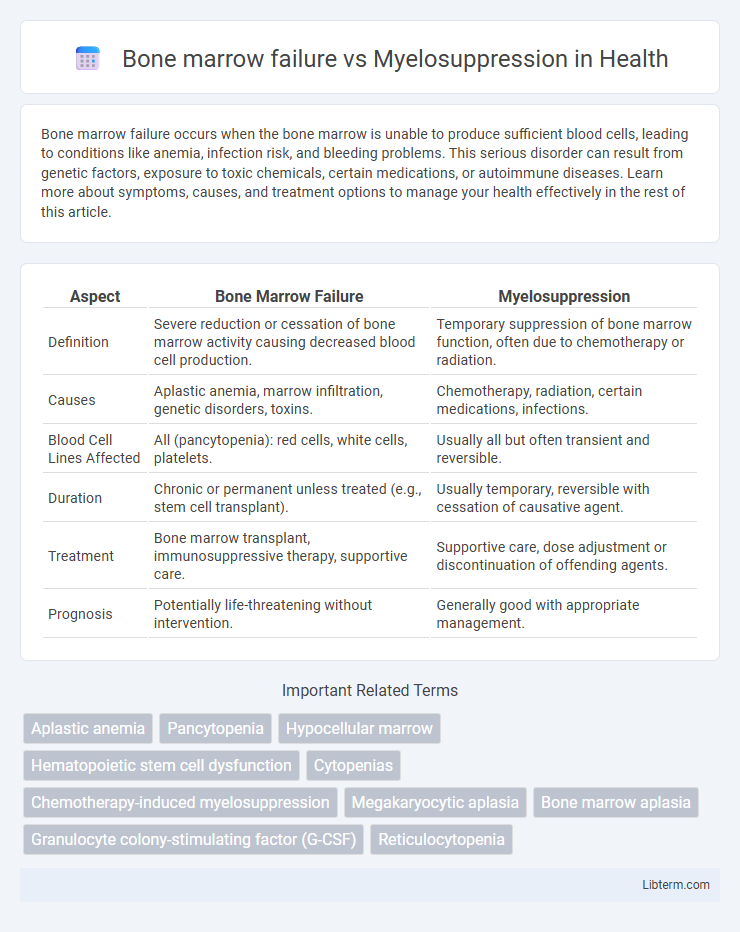
| Aspect | Bone Marrow Failure | Myelosuppression |
|---|---|---|
| Definition | Severe reduction or cessation of bone marrow activity causing decreased blood cell production. | Temporary suppression of bone marrow function, often due to chemotherapy or radiation. |
| Causes | Aplastic anemia, marrow infiltration, genetic disorders, toxins. | Chemotherapy, radiation, certain medications, infections. |
| Blood Cell Lines Affected | All (pancytopenia): red cells, white cells, platelets. | Usually all but often transient and reversible. |
| Duration | Chronic or permanent unless treated (e.g., stem cell transplant). | Usually temporary, reversible with cessation of causative agent. |
| Treatment | Bone marrow transplant, immunosuppressive therapy, supportive care. | Supportive care, dose adjustment or discontinuation of offending agents. |
| Prognosis | Potentially life-threatening without intervention. | Generally good with appropriate management. |
Understanding Bone Marrow Failure
Bone marrow failure involves the inability of the marrow to produce adequate blood cells, often caused by genetic disorders, aplastic anemia, or exposure to toxins. Myelosuppression refers to decreased bone marrow activity typically induced by chemotherapy or radiation, leading to reduced blood cell counts but usually reversible. Understanding bone marrow failure requires recognizing its persistent and often progressive nature, necessitating interventions such as stem cell transplantation or immunosuppressive therapy.
Defining Myelosuppression
Myelosuppression is a condition characterized by the decreased production of blood cells due to the suppression of bone marrow activity, often caused by chemotherapy, radiation, or certain medications. Unlike bone marrow failure, which is a primary defect resulting in the inability of the marrow to produce adequate blood cells over time, myelosuppression is typically reversible and dose-dependent. Key clinical features of myelosuppression include neutropenia, anemia, and thrombocytopenia, leading to increased risk of infection, fatigue, and bleeding.
Key Differences Between Bone Marrow Failure and Myelosuppression
Bone marrow failure is a condition characterized by the inability of the marrow to produce sufficient blood cells, often due to genetic disorders, aplastic anemia, or marrow infiltration, whereas myelosuppression refers to the temporary reduction of bone marrow activity typically caused by chemotherapy, radiation, or certain medications. Bone marrow failure leads to persistent pancytopenia and requires interventions like bone marrow transplant or immunosuppressive therapy, while myelosuppression usually resolves with supportive care once the causative agent is discontinued. Diagnostic differentiation involves bone marrow biopsy revealing hypocellularity in failure versus reversible suppression in myelosuppression, with implications for prognosis and treatment strategies.
Causes of Bone Marrow Failure
Bone marrow failure primarily results from genetic disorders like Fanconi anemia, acquired conditions such as aplastic anemia, and exposure to toxins or radiation. Unlike myelosuppression, which is often a reversible side effect of chemotherapy or certain medications, bone marrow failure reflects a more permanent or progressive inability of the marrow to produce healthy blood cells. Identifying specific causes like viral infections (e.g., parvovirus B19) or autoimmune diseases is critical for accurate diagnosis and targeted treatment.
Causes of Myelosuppression
Myelosuppression primarily results from chemotherapy and radiotherapy targeting rapidly dividing cells, leading to decreased bone marrow activity and reduced production of blood cells. Other causes include infections such as viral hepatitis or HIV, autoimmune disorders, and exposure to toxic chemicals or certain medications like antibiotics and anticonvulsants. Differentiating myelosuppression from bone marrow failure is critical, as myelosuppression is often reversible upon cessation of the causative agent, whereas bone marrow failure involves intrinsic marrow pathology or genetic abnormalities.
Clinical Manifestations: Comparing Symptoms
Bone marrow failure typically presents with pancytopenia, characterized by anemia, leukopenia, and thrombocytopenia, leading to symptoms such as fatigue, frequent infections, and bleeding tendencies. Myelosuppression, often caused by chemotherapy or radiation, primarily results in neutropenia and thrombocytopenia, increasing susceptibility to infections and bleeding but generally developing more rapidly than bone marrow failure. Both conditions exhibit overlapping clinical manifestations, yet the chronicity and underlying causes differ, necessitating distinct diagnostic and therapeutic approaches.
Diagnostic Approaches for Both Conditions
Bone marrow failure is diagnosed through bone marrow biopsy, peripheral blood counts, and cytogenetic analysis to identify underlying causes such as aplastic anemia or myelodysplastic syndrome, while myelosuppression diagnosis primarily relies on evaluating recent chemotherapy exposure, complete blood count trends, and bone marrow cellularity assessment. Flow cytometry and molecular testing help differentiate inherited marrow failure syndromes from acquired conditions, whereas myelosuppression is confirmed by reversible marrow suppression patterns post-chemotherapy or radiation therapy. Advanced imaging like MRI may assist in assessing marrow infiltration in bone marrow failure but is less commonly required for myelosuppression diagnosis.
Treatment Options and Management Strategies
Bone marrow failure requires interventions such as hematopoietic stem cell transplantation, immunosuppressive therapy, or growth factor administration to restore marrow function and improve blood cell production. Myelosuppression management primarily involves dose adjustment of chemotherapy agents, supportive care with transfusions and growth factors like G-CSF to stimulate granulocyte recovery. Both conditions benefit from multidisciplinary monitoring to prevent complications such as infections and bleeding.
Prognosis and Outcomes
Bone marrow failure often results in persistent cytopenias with poor prognosis due to irreversible hematopoietic stem cell damage, frequently requiring bone marrow transplantation for improved outcomes. Myelosuppression, commonly a reversible condition induced by chemotherapy or radiation, typically shows better recovery rates with supportive care and dose adjustments, though severe cases may lead to complications such as infections or bleeding. Prognosis in bone marrow failure is generally more guarded compared to myelosuppression, reflecting the underlying chronicity and treatment complexity of marrow failure syndromes.
Preventive Measures and Patient Monitoring
Preventive measures for bone marrow failure emphasize early detection of genetic disorders and avoidance of toxic exposures like radiation and chemotherapy, while myelosuppression prevention targets dose adjustment and supportive care during cytotoxic treatments. Patient monitoring for bone marrow failure requires regular complete blood counts (CBC) and bone marrow biopsies to assess cellularity and detect aplasia or dysplasia. In myelosuppression, frequent CBC monitoring tracks declining blood cell lines, guiding interventions such as growth factor administration and infection surveillance to reduce complications.
Bone marrow failure Infographic
 libterm.com
libterm.com