Tumor suppressor genes play a crucial role in regulating cell growth and preventing uncontrolled cell division that leads to cancer. Mutations or deletions in these genes can disrupt normal cellular functions, increasing the risk of tumor development and progression. Explore the rest of the article to understand how tumor suppressor genes impact cancer prevention and treatment strategies.
Table of Comparison
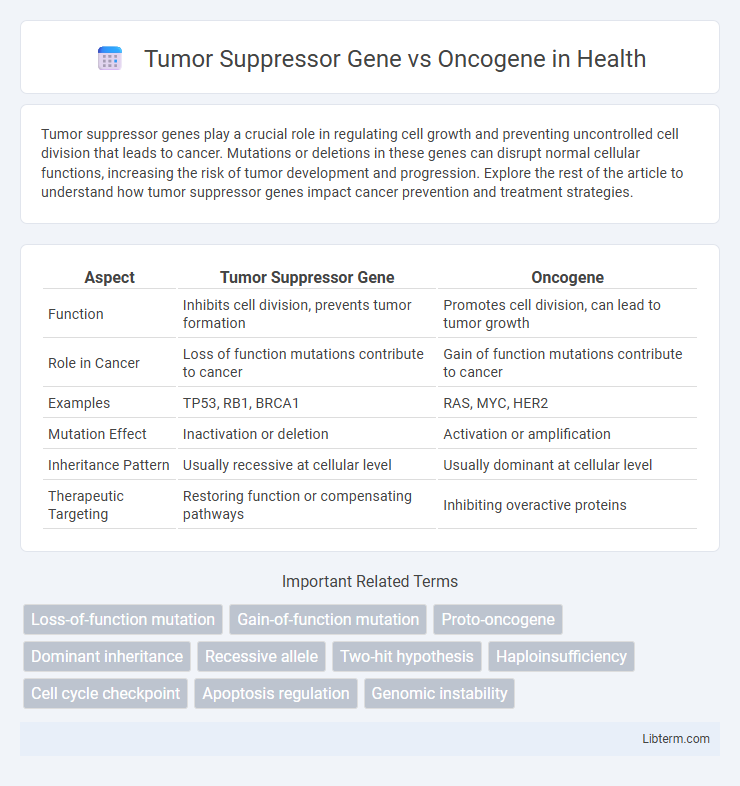
| Aspect | Tumor Suppressor Gene | Oncogene |
|---|---|---|
| Function | Inhibits cell division, prevents tumor formation | Promotes cell division, can lead to tumor growth |
| Role in Cancer | Loss of function mutations contribute to cancer | Gain of function mutations contribute to cancer |
| Examples | TP53, RB1, BRCA1 | RAS, MYC, HER2 |
| Mutation Effect | Inactivation or deletion | Activation or amplification |
| Inheritance Pattern | Usually recessive at cellular level | Usually dominant at cellular level |
| Therapeutic Targeting | Restoring function or compensating pathways | Inhibiting overactive proteins |
Introduction to Tumor Suppressor Genes and Oncogenes
Tumor suppressor genes regulate cell growth by inhibiting uncontrolled proliferation and promoting DNA repair or apoptosis when genetic damage occurs. Oncogenes arise from mutated proto-oncogenes and drive cancer development by continuously signaling cells to divide and evade normal growth controls. Understanding the distinct roles of tumor suppressor genes and oncogenes is crucial for targeted cancer therapies and molecular diagnostics.
Definition and Function of Tumor Suppressor Genes
Tumor suppressor genes are essential components of cellular regulation that encode proteins responsible for inhibiting uncontrolled cell growth and maintaining genomic stability. These genes function by repairing DNA damage, inducing apoptosis in damaged cells, and controlling cell cycle checkpoints to prevent tumor formation. Unlike oncogenes, which promote cell proliferation, tumor suppressor genes act as brakes on cell division, and mutations in these genes often lead to loss of function, contributing to cancer development.
Oncogenes: Meaning and Biological Role
Oncogenes are mutated or overexpressed versions of normal genes called proto-oncogenes that drive uncontrolled cell division and contribute to cancer development. These genes encode proteins involved in essential cellular processes such as growth factor signaling, cell cycle regulation, and apoptosis inhibition. The activation of oncogenes leads to persistent proliferative signaling, evasion of programmed cell death, and enhanced survival, promoting tumor progression.
Key Molecular Differences Between Tumor Suppressor Genes and Oncogenes
Tumor suppressor genes encode proteins that regulate cell cycle checkpoints, DNA repair, and apoptosis, preventing uncontrolled cell proliferation, whereas oncogenes arise from mutated proto-oncogenes that promote cell growth and division. Loss-of-function mutations in tumor suppressor genes, such as TP53 or RB1, result in the inability to inhibit tumor formation, while gain-of-function mutations or amplifications in oncogenes like MYC or RAS lead to enhanced oncogenic signaling. The molecular distinction lies in tumor suppressor gene inactivation requiring both alleles to be affected (Knudson's two-hit hypothesis), whereas oncogene activation often results from a single allele alteration.
Mechanisms of Mutation and Activation
Tumor suppressor genes undergo loss-of-function mutations, often through deletions, nonsense mutations, or promoter hypermethylation, leading to the inactivation of proteins that regulate cell cycle arrest, DNA repair, and apoptosis. Oncogenes result from gain-of-function mutations such as point mutations, gene amplifications, or chromosomal translocations that cause constitutive activation of signaling pathways promoting cell proliferation and survival. The mutation mechanisms distinguish tumor suppressor genes by requiring two "hits" for inactivation, whereas oncogenes typically require a single activating mutation for oncogenic potential.
Examples of Major Tumor Suppressor Genes
Major tumor suppressor genes include TP53, RB1, and BRCA1, which play critical roles in regulating cell growth and maintaining genomic stability. TP53, often called the "guardian of the genome," controls cell cycle arrest and apoptosis to prevent cancer development. Mutations in RB1 disrupt cell cycle regulation, while BRCA1 mutations are linked to hereditary breast and ovarian cancers.
Prominent Examples of Oncogenes
Prominent examples of oncogenes include the MYC gene, which drives cell proliferation in various cancers, and the RAS gene family, frequently mutated in pancreatic, lung, and colorectal cancers. The HER2 gene, often amplified in breast cancer, promotes aggressive tumor growth by encoding a receptor tyrosine kinase involved in cell signaling. Unlike tumor suppressor genes that inhibit tumor formation, these oncogenes facilitate uncontrolled cell division and survival, contributing directly to oncogenesis.
Implications in Cancer Development and Progression
Tumor suppressor genes regulate cell division and repair DNA damage, preventing uncontrolled cell growth, while oncogenes promote cell proliferation and survival, often driving cancer development when mutated or overexpressed. Loss of function in tumor suppressor genes such as TP53 or RB1 leads to failure in cell cycle control and genomic instability, contributing to tumor progression. Conversely, mutations activating oncogenes like KRAS or MYC result in continuous cell signaling for growth and division, accelerating cancer progression and resistance to apoptosis.
Therapeutic Strategies Targeting Tumor Suppressor Genes and Oncogenes
Therapeutic strategies targeting tumor suppressor genes primarily involve restoring their normal function through gene therapy or drugs that reactivate mutated genes like p53, thereby promoting cell cycle arrest and apoptosis. In contrast, treatments targeting oncogenes focus on inhibiting overactive proteins with small molecule inhibitors or monoclonal antibodies, such as tyrosine kinase inhibitors in cancers driven by EGFR or HER2 amplification. Combining these approaches enhances treatment efficacy by simultaneously suppressing oncogenic pathways and reinstating tumor suppression mechanisms.
Future Directions in Cancer Research and Precision Medicine
Future directions in cancer research emphasize targeting tumor suppressor genes and oncogenes to develop more effective precision medicine therapies. Advances in CRISPR gene editing and single-cell sequencing enable precise identification and modulation of these critical genetic factors, enhancing personalized treatment strategies. Integrating multi-omics data with artificial intelligence accelerates the discovery of novel biomarkers and therapeutic targets, revolutionizing cancer prognosis and drug development.
Tumor Suppressor Gene Infographic
 libterm.com
libterm.com