Fetal hemoglobin (HbF) is a specialized form of hemoglobin found primarily in fetuses and newborns that efficiently binds oxygen to support development in low-oxygen environments. Its unique structure allows higher oxygen affinity compared to adult hemoglobin, facilitating oxygen transfer from mother to fetus. Explore this article to learn more about the critical role and potential clinical applications of fetal hemoglobin in treating blood disorders.
Table of Comparison
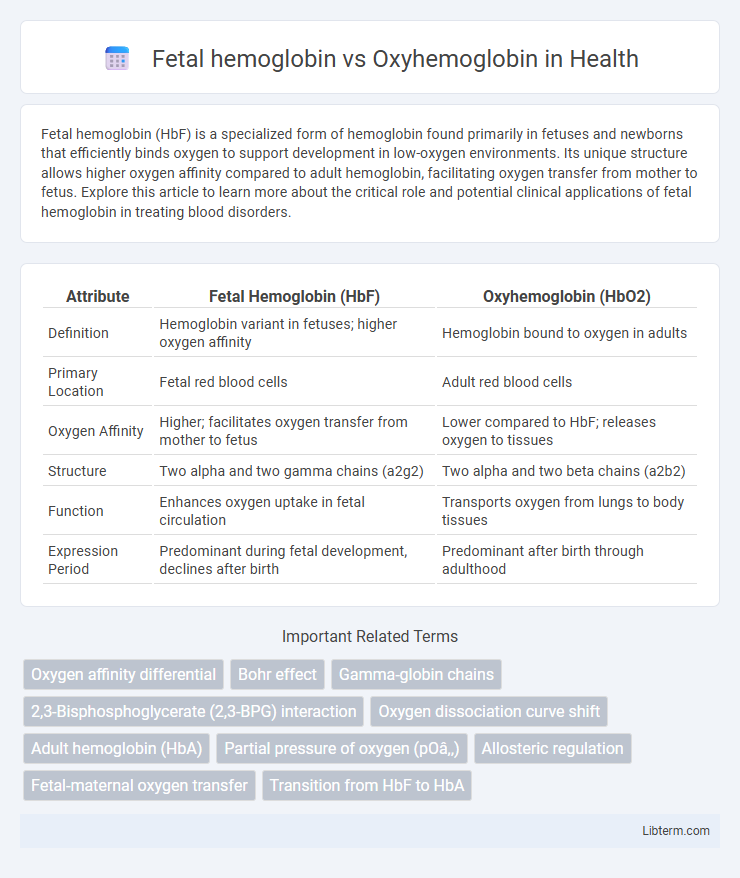
| Attribute | Fetal Hemoglobin (HbF) | Oxyhemoglobin (HbO2) |
|---|---|---|
| Definition | Hemoglobin variant in fetuses; higher oxygen affinity | Hemoglobin bound to oxygen in adults |
| Primary Location | Fetal red blood cells | Adult red blood cells |
| Oxygen Affinity | Higher; facilitates oxygen transfer from mother to fetus | Lower compared to HbF; releases oxygen to tissues |
| Structure | Two alpha and two gamma chains (a2g2) | Two alpha and two beta chains (a2b2) |
| Function | Enhances oxygen uptake in fetal circulation | Transports oxygen from lungs to body tissues |
| Expression Period | Predominant during fetal development, declines after birth | Predominant after birth through adulthood |
Introduction to Hemoglobin Types
Fetal hemoglobin (HbF) and oxyhemoglobin (HbO2) represent distinct functional states of hemoglobin, crucial for oxygen transport in humans. HbF, predominant during fetal development, exhibits a higher affinity for oxygen compared to adult hemoglobin, enabling efficient oxygen transfer from maternal to fetal blood. Oxyhemoglobin forms when adult hemoglobin (HbA) binds oxygen in the lungs, facilitating oxygen delivery to tissues throughout the body.
Structural Differences: Fetal vs Oxyhemoglobin
Fetal hemoglobin (HbF) consists of two alpha and two gamma subunits, whereas oxyhemoglobin contains two alpha and two beta subunits, leading to differences in oxygen affinity and quaternary structure. The gamma chains in HbF reduce its affinity for 2,3-Bisphosphoglycerate (2,3-BPG), stabilizing the oxygen-bound form and facilitating oxygen transfer from maternal oxyhemoglobin to fetal blood. Structural variations in the globin chains result in distinct conformational states that optimize oxygen binding and release under physiological conditions specific to fetal development.
Oxygen Binding Affinity Comparison
Fetal hemoglobin (HbF) exhibits a significantly higher oxygen binding affinity than adult oxyhemoglobin (HbA), enabling efficient oxygen transfer from maternal blood to the fetus. HbF's reduced affinity for 2,3-bisphosphoglycerate (2,3-BPG) stabilizes its oxygen-bound state, thus facilitating enhanced oxygen uptake in hypoxic fetal environments. This distinct oxygen affinity difference is critical for maintaining adequate fetal oxygenation during gestation.
Role in Oxygen Transport
Fetal hemoglobin (HbF) has a higher affinity for oxygen compared to oxyhemoglobin (HbO2), enabling efficient oxygen transfer from maternal to fetal blood across the placenta. HbF's unique gamma globin chains facilitate this increased oxygen-binding capacity at lower partial pressures, crucial for fetal development in a relatively hypoxic environment. In contrast, oxyhemoglobin in adult blood efficiently releases oxygen to tissues, optimizing oxygen delivery and metabolic function post-birth.
Expression During Developmental Stages
Fetal hemoglobin (HbF) is predominantly expressed during the prenatal developmental stages, providing higher oxygen affinity to facilitate efficient oxygen transfer from the maternal to fetal blood. After birth, the expression of HbF gradually declines, while adult oxyhemoglobin (HbA) becomes the dominant form in red blood cells, adapting to postnatal oxygen transport requirements. This switch in hemoglobin expression is regulated by complex genetic mechanisms involving the gamma-globin gene silencing and beta-globin gene activation.
Genetic Regulation and Switching
Fetal hemoglobin (HbF) consists primarily of two alpha and two gamma globin chains, regulated by the gamma-globin genes located on chromosome 11, whereas oxyhemoglobin refers to adult hemoglobin (HbA) bound to oxygen, composed of alpha and beta chains. The genetic switch from gamma-globin to beta-globin gene expression occurs around birth, controlled by regulatory elements such as the locus control region (LCR) and transcription factors including BCL11A and KLF1. Understanding the molecular mechanisms behind this hemoglobin switching aids in developing treatments for hemoglobinopathies like sickle cell disease and beta-thalassemia by reactivating HbF expression.
Clinical Significance & Disorders
Fetal hemoglobin (HbF) exhibits a higher affinity for oxygen compared to adult oxyhemoglobin (HbA), facilitating efficient oxygen transfer from mother to fetus in utero. Clinically, elevated levels of HbF are beneficial in disorders like sickle cell disease and beta-thalassemia, where increased HbF reduces severity by inhibiting hemoglobin S polymerization and improving oxygen delivery. Abnormal persistence or reduction of HbF can indicate hematologic conditions such as hereditary persistence of fetal hemoglobin (HPFH) or ineffective erythropoiesis, impacting diagnostic and therapeutic strategies.
Diagnostic Uses in Medicine
Fetal hemoglobin (HbF) and oxyhemoglobin serve distinct roles in medical diagnostics, with HbF levels crucial for identifying hemoglobinopathies such as beta-thalassemia and sickle cell disease in neonates and adults. Measuring oxyhemoglobin saturation via pulse oximetry provides real-time assessment of oxygen delivery and respiratory function in critical care and pulmonary medicine. Advanced techniques like high-performance liquid chromatography (HPLC) and capillary electrophoresis quantify HbF concentrations, guiding transfusion therapy and monitoring disease progression.
Therapeutic Applications and Research
Fetal hemoglobin (HbF) exhibits a higher affinity for oxygen compared to adult oxyhemoglobin (HbA), making it a critical target in therapeutic research for sickle cell disease and beta-thalassemia, where increased HbF levels reduce disease severity by inhibiting sickling of red blood cells. Pharmacological agents such as hydroxyurea and gene editing technologies aim to reactivate HbF production, offering promising avenues for treatment. Ongoing clinical trials investigate CRISPR-Cas9 and other genomic approaches to sustainably elevate HbF levels, highlighting their potential in regenerative medicine and inherited hemoglobinopathy management.
Summary: Key Distinctions and Implications
Fetal hemoglobin (HbF) has a higher affinity for oxygen than oxyhemoglobin (HbO2), enabling efficient oxygen transfer from maternal to fetal blood across the placenta. Structurally, HbF consists of two alpha and two gamma chains, while adult oxyhemoglobin contains two alpha and two beta chains, influencing their oxygen-binding properties. This distinction in oxygen affinity is critical for fetal development, ensuring adequate oxygen supply despite lower maternal oxygen levels.
Fetal hemoglobin Infographic
 libterm.com
libterm.com