Placebo control is a fundamental method in clinical trials used to measure the true effect of a treatment by comparing it to an inactive substance. This approach minimizes bias and ensures that outcomes are attributed to the treatment rather than participants' expectations. Explore the article to understand how placebo controls enhance the reliability of medical research.
Table of Comparison
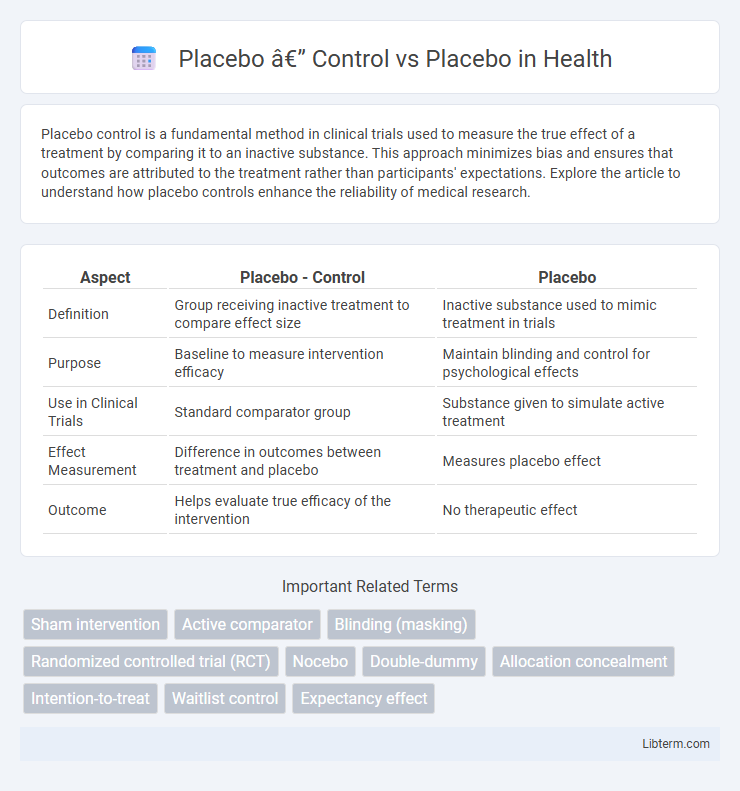
| Aspect | Placebo - Control | Placebo |
|---|---|---|
| Definition | Group receiving inactive treatment to compare effect size | Inactive substance used to mimic treatment in trials |
| Purpose | Baseline to measure intervention efficacy | Maintain blinding and control for psychological effects |
| Use in Clinical Trials | Standard comparator group | Substance given to simulate active treatment |
| Effect Measurement | Difference in outcomes between treatment and placebo | Measures placebo effect |
| Outcome | Helps evaluate true efficacy of the intervention | No therapeutic effect |
Introduction to Placebo in Clinical Research
Placebo in clinical research refers to an inert substance or treatment designed to mimic the experimental intervention without containing active therapeutic ingredients. It serves as a control to isolate the true effects of the tested drug by accounting for psychological and physiological responses unrelated to the treatment's efficacy. The placebo effect highlights the significance of patient perception in clinical outcomes, emphasizing the necessity of placebo controls to validate the effectiveness of new medical therapies.
Defining Control Groups and Placebo Groups
Control groups in clinical trials serve as the baseline to compare the effects of the treatment, receiving no active intervention or a standard treatment. Placebo groups receive an inactive substance designed to mimic the treatment without therapeutic effect, ensuring blinding and minimizing bias. Defining these groups precisely is critical for maintaining study validity and accurately assessing the placebo effect versus the actual treatment outcome.
The Role of Placebo in Scientific Studies
The role of placebo in scientific studies is critical for isolating the true effects of a treatment by comparing outcomes between the control group receiving placebo and the experimental group. Placebo control helps eliminate bias, ensuring that observed benefits are due to the active intervention rather than psychological or physiological responses to receiving any treatment. This methodology enhances the reliability and validity of clinical trial results, making it a cornerstone in drug development and therapeutic research.
Types of Control Groups: Placebo vs. Active Control
Placebo control groups receive an inert substance to measure the psychological and physiological effects of expectation, serving as a baseline to assess the efficacy of a new treatment. Active control groups are administered an established treatment, allowing researchers to compare the new intervention's effectiveness against a standard therapy. This distinction between placebo and active control types ensures accurate evaluation of therapeutic benefits and helps in distinguishing true drug effects from placebo responses.
How Placebos Influence Study Outcomes
Placebos significantly impact study outcomes by serving as a control to measure the true effect of a treatment against psychological and physiological responses triggered by patients' expectations. In randomized controlled trials, placebo groups help isolate the specific effects of an intervention from placebo effects, which can include symptom improvement due to belief in treatment. Understanding placebo influence is critical for accurately assessing drug efficacy and minimizing bias in clinical research.
Designing Robust Placebo-Controlled Trials
Designing robust placebo-controlled trials requires careful consideration of randomization methods, blinding procedures, and appropriate placebo selection to minimize bias and enhance validity. Ensuring the placebo resembles the active treatment in appearance, taste, and administration maintains participant blinding, which is crucial for accurately assessing the treatment's efficacy. Statistical power calculations and predefined endpoints further strengthen the trial's ability to detect meaningful differences between control and placebo groups.
Ethical Considerations of Using Placebos
The ethical considerations of using placebos in clinical trials focus on ensuring informed consent and balancing the need for scientific rigor with patient welfare. Ethical guidelines emphasize that placebos should only be used when no proven effective treatment exists or when withholding treatment poses no significant risk to participants. Transparency, minimizing harm, and maintaining trust between researchers and patients are paramount in placebo-controlled studies to uphold ethical standards in medical research.
Advantages and Limitations of Placebo Controls
Placebo controls in clinical trials provide a baseline to measure the efficacy of a new treatment by eliminating psychological bias and ensuring blinding. Advantages include minimizing placebo effects, enhancing internal validity, and facilitating objective comparison between active and inactive interventions. Limitations involve ethical concerns when withholding effective therapy, potential unblinding due to side effects, and limited applicability in certain conditions where placebo response is minimal or impractical.
Interpreting Results: Placebo Effect vs. True Effect
Interpreting results in placebo-controlled studies involves distinguishing between the placebo effect and the true effect of the treatment. The placebo effect arises from participants' expectations and psychological factors, leading to perceived or actual improvements despite receiving an inert substance. Accurate interpretation requires comparing outcomes in the placebo group to those in the treatment group to isolate the treatment's specific efficacy from non-specific psychological influences.
Future Trends in Placebo and Control Research
Future trends in placebo and control research emphasize personalized placebo effects through genetic and neurobiological profiling, enhancing treatment efficacy prediction. Advances in digital health technologies enable real-time monitoring of placebo responses, facilitating adaptive clinical trial designs. Integration of artificial intelligence optimizes placebo control methodologies, improving the accuracy and reliability of therapeutic outcome assessments.
Placebo — Control Infographic
 libterm.com
libterm.com