Toxicity refers to the degree to which a substance can cause harm to living organisms, impacting health and the environment. Understanding toxicity levels is crucial for safe handling of chemicals and preventing adverse effects on your body and surroundings. Explore the rest of this article to learn more about how toxicity affects you and ways to minimize exposure.
Table of Comparison
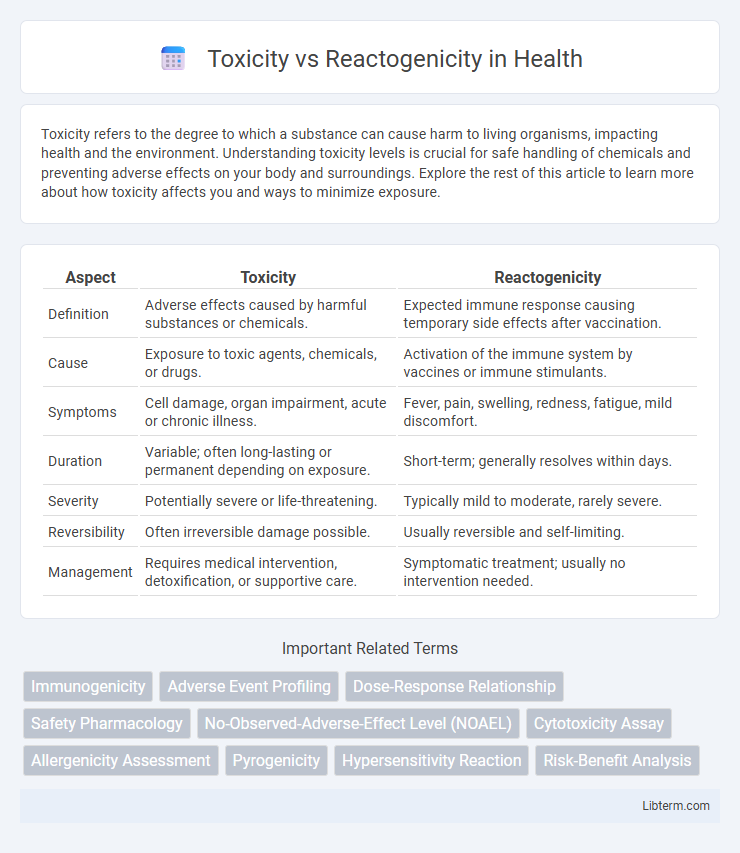
| Aspect | Toxicity | Reactogenicity |
|---|---|---|
| Definition | Adverse effects caused by harmful substances or chemicals. | Expected immune response causing temporary side effects after vaccination. |
| Cause | Exposure to toxic agents, chemicals, or drugs. | Activation of the immune system by vaccines or immune stimulants. |
| Symptoms | Cell damage, organ impairment, acute or chronic illness. | Fever, pain, swelling, redness, fatigue, mild discomfort. |
| Duration | Variable; often long-lasting or permanent depending on exposure. | Short-term; generally resolves within days. |
| Severity | Potentially severe or life-threatening. | Typically mild to moderate, rarely severe. |
| Reversibility | Often irreversible damage possible. | Usually reversible and self-limiting. |
| Management | Requires medical intervention, detoxification, or supportive care. | Symptomatic treatment; usually no intervention needed. |
Understanding Toxicity and Reactogenicity
Toxicity refers to the degree to which a substance can cause harmful effects on an organism, often manifesting as cellular damage, organ dysfunction, or adverse systemic responses. Reactogenicity describes the physical manifestation of the body's inflammatory response to a vaccine or drug, including symptoms like fever, pain, and swelling at the injection site. Understanding the distinction between toxicity, which implies potential long-term harm, and reactogenicity, typically representing temporary immune activation, is critical for evaluating drug safety profiles and vaccine tolerability.
Key Differences Between Toxicity and Reactogenicity
Toxicity refers to the degree to which a substance can cause harmful effects on an organism, often involving long-term or severe damage at cellular or systemic levels, whereas reactogenicity describes the capacity of a vaccine or drug to produce common, temporary adverse reactions like inflammation or fever at the site of administration. Key differences include toxicity's potential for lasting harm and systemic impact, contrasted with reactogenicity's usually mild, transient symptoms related to the immune response. Understanding these distinctions is critical for evaluating drug safety profiles and managing patient expectations during clinical trials and therapeutic use.
Mechanisms Underlying Toxicity
Toxicity involves harmful effects caused by chemical or biological agents disrupting cellular homeostasis through mechanisms like oxidative stress, mitochondrial dysfunction, and induction of apoptosis. In contrast, reactogenicity primarily results from immune system activation leading to inflammatory responses such as cytokine release and localized tissue irritation. Understanding these distinct mechanisms is critical for developing safer pharmaceuticals and vaccines with minimized adverse effects.
Mechanisms Behind Reactogenicity
Reactogenicity arises from the innate immune response triggered by vaccine components, such as adjuvants and antigens, which stimulate the release of pro-inflammatory cytokines like interleukin-6 and tumor necrosis factor-alpha. This activation of immune cells leads to localized symptoms such as pain, redness, and swelling at the injection site, as well as systemic effects like fever and malaise. In contrast, toxicity involves harmful effects caused by excessive or off-target immune reactions, cellular damage, or toxic substances beyond the controlled inflammatory response characteristic of reactogenicity.
Short-Term vs Long-Term Effects
Toxicity refers to the harmful effects of a substance that can manifest either shortly after exposure or accumulate over time, leading to chronic health issues. Reactogenicity describes the immediate and transient inflammatory responses, such as fever or soreness, typically resolving within days. While reactogenicity indicates short-term immune activation without lasting damage, toxicity can involve both acute reactions and serious long-term consequences depending on the dose and duration of exposure.
Examples in Vaccines and Medications
Toxicity in vaccines and medications refers to harmful effects caused by the substance itself, such as liver damage from acetaminophen overdose or neurotoxicity from high doses of certain chemotherapy drugs. Reactogenicity describes the expected, transient inflammatory responses like fever, injection site pain, or swelling often seen after influenza vaccines or the COVID-19 mRNA vaccines. Distinguishing toxicity from reactogenicity is crucial for evaluating vaccine safety profiles, as toxicity entails direct adverse biological damage while reactogenicity involves normal immune activation symptoms.
Measuring and Assessing Toxicity
Measuring toxicity involves evaluating adverse effects on biological systems through clinical observations, biochemical assays, and histopathological analysis to determine safe dosage levels. Reactogenicity assessment focuses on the immediate, short-term immune responses such as inflammation, fever, or injection site reactions, primarily using patient self-reports and biomarker quantification. Objective toxicity evaluation requires standardized protocols, including in vitro cytotoxicity tests and in vivo animal studies, to distinguish harmful compound effects from expected reactogenic responses.
Assessing Reactogenicity in Clinical Trials
Assessing reactogenicity in clinical trials involves monitoring common, expected adverse reactions such as pain, swelling, fever, and fatigue that occur shortly after vaccination. This evaluation helps distinguish transient immune responses from more severe toxic effects, ensuring vaccine safety profiles are accurately characterized. Standardized grading scales and patient diaries facilitate consistent data collection and interpretation of reactogenicity across trial participants.
Safety Implications for Patients
Toxicity refers to the harmful effects caused by exposure to harmful substances, leading to cellular damage or organ dysfunction, whereas reactogenicity describes the common, transient inflammatory responses to vaccines or medications, such as fever or injection site pain. Understanding the distinction is crucial for patient safety, as toxicity may result in serious adverse outcomes requiring medical intervention, while reactogenicity typically signals an expected immune response with minimal long-term risk. Clinicians must carefully monitor both to balance therapeutic benefits against potential safety risks, ensuring informed decision-making and optimal patient care.
Reducing Risks: Strategies and Innovations
Reducing risks associated with toxicity and reactogenicity involves advanced drug formulation techniques such as controlled-release delivery systems and nanoparticle carriers to minimize adverse effects. Innovations in predictive toxicology using in vitro models and AI-driven simulations enable early identification of potential toxic responses, enhancing safety profiles. Personalized medicine approaches, including genetic screening, help tailor treatments to individual susceptibility, substantially lowering reactogenicity risks.
Toxicity Infographic
 libterm.com
libterm.com