Seroprevalence measures the level of a specific antibody in a population, indicating how widespread a particular infection has been. Understanding seroprevalence helps track immunity and exposure rates, guiding public health decisions and vaccine strategies. Explore the rest of the article to learn how seroprevalence data impacts disease control and prevention efforts.
Table of Comparison
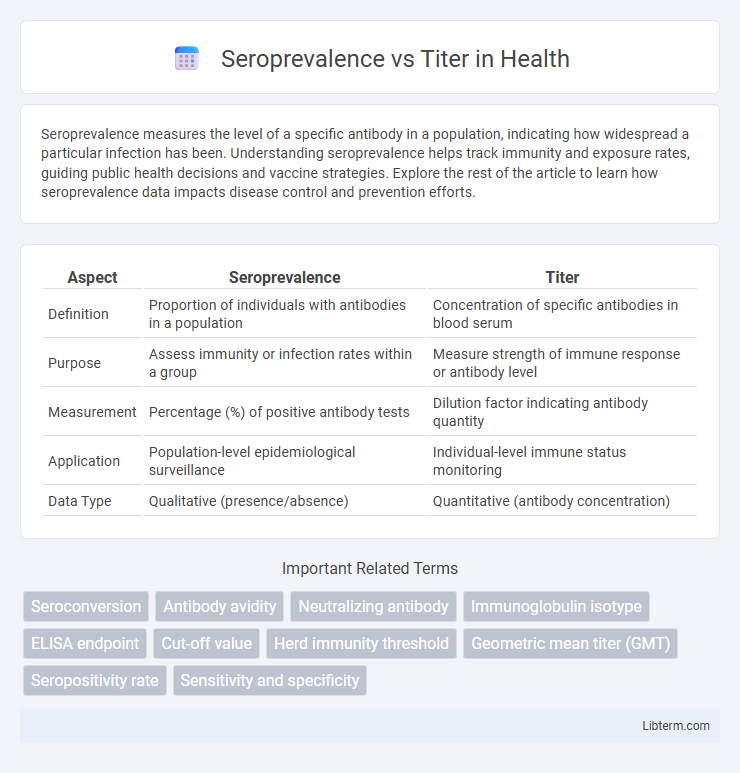
| Aspect | Seroprevalence | Titer |
|---|---|---|
| Definition | Proportion of individuals with antibodies in a population | Concentration of specific antibodies in blood serum |
| Purpose | Assess immunity or infection rates within a group | Measure strength of immune response or antibody level |
| Measurement | Percentage (%) of positive antibody tests | Dilution factor indicating antibody quantity |
| Application | Population-level epidemiological surveillance | Individual-level immune status monitoring |
| Data Type | Qualitative (presence/absence) | Quantitative (antibody concentration) |
Understanding Seroprevalence: Definition and Importance
Seroprevalence measures the proportion of individuals in a population with antibodies against a specific pathogen, indicating prior exposure or immunity levels. It is essential for assessing population immunity, guiding public health interventions, and evaluating vaccine effectiveness. Contrarily, antibody titer quantifies the concentration of antibodies in an individual's blood, providing a detailed immune response measure rather than population-level insights.
Antibody Titer Explained: What Does It Measure?
Antibody titer measures the concentration of specific antibodies in the blood, indicating the strength of an immune response to infections or vaccines. Seroprevalence estimates the proportion of a population with detectable antibodies, reflecting past exposure or immunity. Unlike seroprevalence which is population-based, antibody titer quantifies individual immune status by measuring antibody levels.
Key Differences Between Seroprevalence and Titer
Seroprevalence refers to the proportion of individuals in a population who have detectable antibodies against a specific pathogen, indicating past exposure or infection, while titer measures the concentration or level of antibodies in an individual's blood. Seroprevalence data is used in epidemiology to assess immunity or infection rates within communities, whereas titer provides quantitative information essential for evaluating individual immune response or vaccine efficacy. The key difference lies in seroprevalence representing a population-level prevalence metric, and titer reflecting a precise antibody measurement at the individual level.
Seroprevalence in Epidemiological Studies
Seroprevalence measures the proportion of individuals in a population with detectable antibodies against a specific pathogen, providing crucial data on infection spread and population immunity in epidemiological studies. It helps estimate true infection rates, including asymptomatic cases, which titer levels alone cannot fully represent. These insights guide public health strategies for vaccination coverage and outbreak containment.
Clinical Applications of Antibody Titers
Antibody titers measure the concentration of specific antibodies in the blood, providing essential data for assessing immunity levels after vaccination or infection. Seroprevalence indicates the proportion of individuals in a population with detectable antibodies, reflecting exposure rates to infectious agents. Clinically, antibody titer assessments guide decisions on booster vaccinations, evaluate immune response in immunocompromised patients, and assist in diagnosing current or past infections.
Sampling Methods for Measuring Seroprevalence
Seroprevalence is measured by detecting the presence of specific antibodies in a population, while titer quantifies the concentration of those antibodies in an individual sample. Sampling methods such as random sampling, stratified sampling, and cluster sampling are critical for accurately estimating seroprevalence in diverse populations, ensuring representation across demographic variables like age, sex, and geographic location. Proper choice of sampling techniques directly influences the reliability of seroprevalence studies used in epidemiological surveillance and public health decision-making.
Laboratory Techniques for Titer Determination
Seroprevalence measures the proportion of a population with detectable specific antibodies, while titer quantifies antibody concentration in an individual serum sample. Laboratory techniques for titer determination include enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), and neutralization tests, which provide quantitative data by serially diluting serum and detecting the lowest concentration that reacts with the antigen. These methods are critical for assessing immune response intensity and guiding epidemiological studies.
Interpreting Seroprevalence and Titer Data
Seroprevalence measures the proportion of individuals in a population with detectable antibodies, indicating prior exposure or vaccination, while titer quantifies the concentration of those antibodies in an individual's blood. Interpreting seroprevalence data helps assess population-level immunity and disease spread, whereas titer values provide insight into the strength and durability of an individual's immune response. Accurate analysis requires considering factors like assay sensitivity, antibody waning, and the timing of sample collection relative to infection or vaccination.
Factors Influencing Seroprevalence vs Titer Results
Seroprevalence estimates the proportion of individuals with detectable antibodies in a population, whereas titer quantifies the specific concentration of antibodies in an individual's blood. Factors influencing seroprevalence include population immunity levels, vaccination coverage, and the sensitivity and specificity of serological assays. In contrast, titer results are affected by the timing of sample collection post-exposure, individual immune response variability, and assay calibration standards.
Seroprevalence and Titer in Public Health Policy
Seroprevalence measures the proportion of a population with detectable antibodies against a specific pathogen, providing critical data on immunity levels and past infection rates essential for public health surveillance. Titer quantifies antibody concentration in individual blood samples, offering insights into immune response strength and vaccine efficacy critical for tailoring immunization strategies. Integrating seroprevalence and titer data informs public health policy by identifying immunity gaps, guiding vaccine distribution, and evaluating herd immunity thresholds to control disease outbreaks.
Seroprevalence Infographic
 libterm.com
libterm.com