Isobaric processes occur at constant pressure, playing a crucial role in thermodynamics and engineering applications such as heating and cooling systems. Understanding how volume and temperature change under isobaric conditions can enhance your grasp of physical and chemical reactions. Explore the rest of the article to learn more about isobaric processes and their practical significance.
Table of Comparison
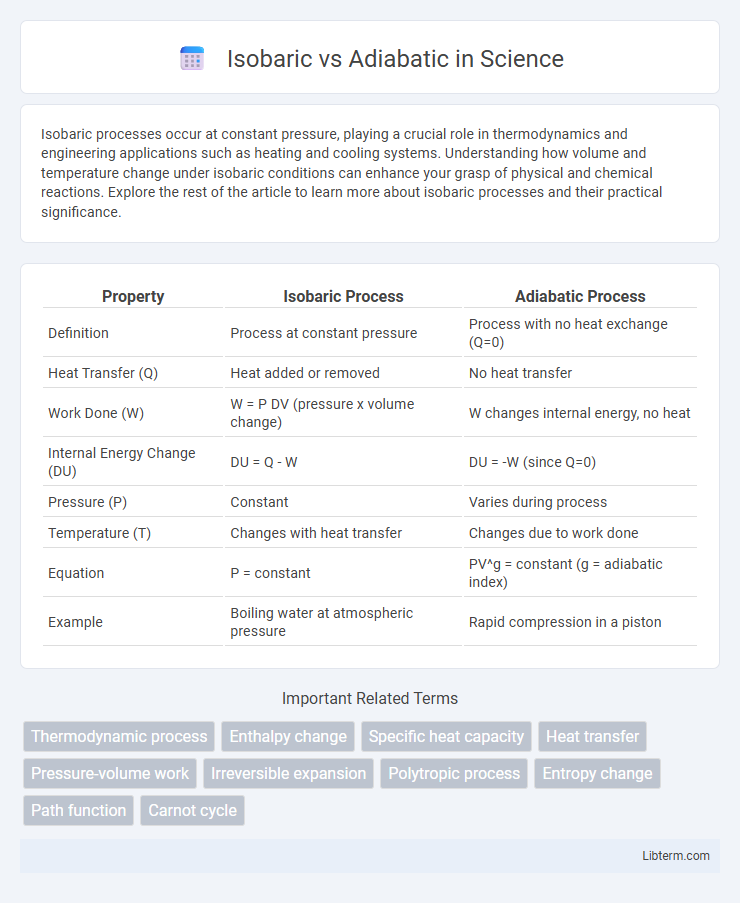
| Property | Isobaric Process | Adiabatic Process |
|---|---|---|
| Definition | Process at constant pressure | Process with no heat exchange (Q=0) |
| Heat Transfer (Q) | Heat added or removed | No heat transfer |
| Work Done (W) | W = P DV (pressure x volume change) | W changes internal energy, no heat |
| Internal Energy Change (DU) | DU = Q - W | DU = -W (since Q=0) |
| Pressure (P) | Constant | Varies during process |
| Temperature (T) | Changes with heat transfer | Changes due to work done |
| Equation | P = constant | PV^g = constant (g = adiabatic index) |
| Example | Boiling water at atmospheric pressure | Rapid compression in a piston |
Introduction to Isobaric and Adiabatic Processes
Isobaric processes occur at constant pressure, allowing the volume of a gas to change while heat is transferred to or from the system. Adiabatic processes involve no heat exchange, where all energy transfer results in a change in the internal energy, typically causing temperature and pressure variations. Understanding these fundamental thermodynamic processes is essential for applications in engines, refrigeration, and atmospheric science.
Fundamental Thermodynamic Concepts
Isobaric processes occur at constant pressure, allowing heat transfer that changes the system's volume and enthalpy, while adiabatic processes involve no heat exchange, leading to changes in internal energy and temperature solely from work done on or by the system. The distinction between these thermodynamic concepts is critical for analyzing energy interactions, where isobaric conditions imply \( \Delta P = 0 \) and adiabatic conditions imply \( Q = 0 \). Understanding the relationship between pressure, volume, temperature, and heat transfer under these constraints is essential for applications such as engine cycles and refrigeration systems.
Defining Isobaric Process
An isobaric process is a thermodynamic transformation occurring at constant pressure, where the system's volume changes while pressure remains steady. During this process, the heat added to the system does work on the surroundings, leading to a change in internal energy and volume expansion or compression. Understanding the isobaric process is crucial for analyzing heat engines, refrigerators, and various chemical reactions where pressure regulation is essential.
Defining Adiabatic Process
An adiabatic process is defined by the absence of heat exchange between a system and its surroundings, meaning all energy transfer occurs solely through work. In contrast to isobaric processes, where pressure remains constant, adiabatic processes often involve rapid compression or expansion resulting in temperature changes without heat transfer. Common examples include the compression of gases in engines and atmospheric processes where insulation or speed prevents heat exchange.
Key Differences: Isobaric vs Adiabatic
Isobaric processes occur at constant pressure, allowing heat exchange with the surroundings to maintain pressure equilibrium, whereas adiabatic processes involve no heat transfer, resulting in temperature changes due to internal energy variation. In isobaric conditions, work done by the system equals pressure multiplied by volume change, while in adiabatic conditions, work done leads to temperature and pressure changes without heat input or output. These fundamental differences significantly impact thermodynamic analysis and applications in engines, compressors, and insulation systems.
Equations Governing Each Process
Isobaric processes follow the equation \( Q = \Delta U + P \Delta V \), where pressure \( P \) remains constant and heat exchange \( Q \) changes internal energy \( \Delta U \) and volume \( \Delta V \). In adiabatic processes, the heat transfer \( Q = 0 \) leads to \( P V^\gamma = \text{constant} \), where \( \gamma \) is the heat capacity ratio \( C_p/C_v \), reflecting no heat exchange and a relationship between pressure \( P \) and volume \( V \). The first law of thermodynamics simplifies to \( \Delta U = W \) in adiabatic conditions, contrasting with the heat-inclusive isobaric equation.
Real-World Examples of Isobaric Processes
Isobaric processes occur at constant pressure and are common in everyday phenomena such as boiling water in an open pot, where heat is added but pressure remains atmospheric. Another real-world example is the expansion of gases in a car engine cylinder during the intake stroke, where volume increases while pressure holds steady. These processes illustrate how energy transfer as heat leads to volume changes without altering pressure, a key concept in thermodynamics and engineering applications.
Real-World Examples of Adiabatic Processes
Adiabatic processes occur without heat exchange, commonly seen in atmospheric phenomena such as rising air parcels cooling and sinking air warming, driving weather patterns. In refrigeration systems, adiabatic expansion allows cooling without external heat transfer, enhancing energy efficiency. Gas turbines also exploit adiabatic compression and expansion to convert thermal energy into mechanical work, optimizing engine performance.
Applications in Engineering and Physics
Isobaric processes, occurring at constant pressure, are critical in thermodynamics for designing engines and HVAC systems, enabling efficient heat transfer and work output under pressure-regulated conditions. Adiabatic processes, characterized by no heat exchange with the environment, are essential in understanding rapid gas expansions and compressions in turbines, compressors, and atmospheric models, where energy changes solely affect internal energy and work done. Engineering applications leverage isobaric processes for steady, controlled thermal management, while adiabatic processes optimize energy transformations in high-speed, insulated systems.
Conclusion: Choosing the Appropriate Process
Selecting between isobaric and adiabatic processes depends on the specific thermodynamic requirements and constraints of the system. Isobaric processes suit applications needing constant pressure with heat exchange, such as in heating or cooling systems, while adiabatic processes are ideal for systems requiring no heat transfer, like gas compression or expansion in turbines. The appropriate choice balances energy efficiency, system design, and operational conditions.
Isobaric Infographic
 libterm.com
libterm.com